Lomitapide mesylate |
| Catalog No.GC36478 |
Le mésylate de lomitapide (AEGR-733; BMS-201038) est un inhibiteur de la protéine microsomale de transfert des triglycérides (MTP) avec une IC50 in vitro de 8nM.
Products are for research use only. Not for human use. We do not sell to patients.
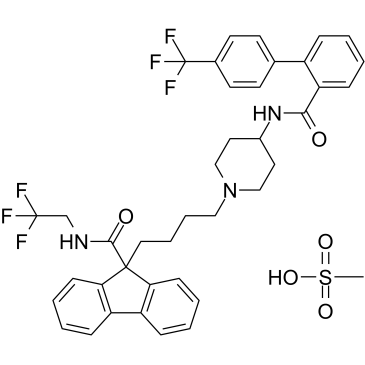
Cas No.: 202914-84-9
Sample solution is provided at 25 µL, 10mM.
Lomitapide mesylate(AEGR-733; BMS-201038) is an inhibitor of microsomal triglyceride-transfer protein (MTP) wtih in vitro IC50 of 8 nM.IC50 value: 8 nM [1]Target: MTP inhibitorLomitapide is a small-molecule, microsomal triglyceride transfer protein (MTP) inhibitor, for the treatment of both familial and primary hypercholesterolemia. Oral, once-daily lomitapide will be targeted at patients resistant to HMG-CoA reductase inhibitors (statins) either due to abnormalities in liver function or to discontinuation because of muscle pain.
[1]. Sulsky R, et al. 5-Carboxamido-1,3,2-dioxaphosphorinanes, potent inhibitors of MTP. Bioorg Med Chem Lett. 2004 Oct 18;14(20):5067-70. [2]. Lomitapide. Am J Cardiovasc Drugs. 2011 Oct 1;11(5):347-52. [3]. Perry CM. Lomitapide: a review of its use in adults with homozygous familial hypercholesterolemia. Am J Cardiovasc Drugs. 2013 Aug;13(4):285-96.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















