(S)-Flurbiprofen |
| Catalog No.GC18022 |
Le (S)-flurbiprofène est un énantiomère actif du flurbiprofène, avec des valeurs IC50 de 0,48 μM et 0,47 μM pour COX-1 et COX-2, respectivement.
Products are for research use only. Not for human use. We do not sell to patients.
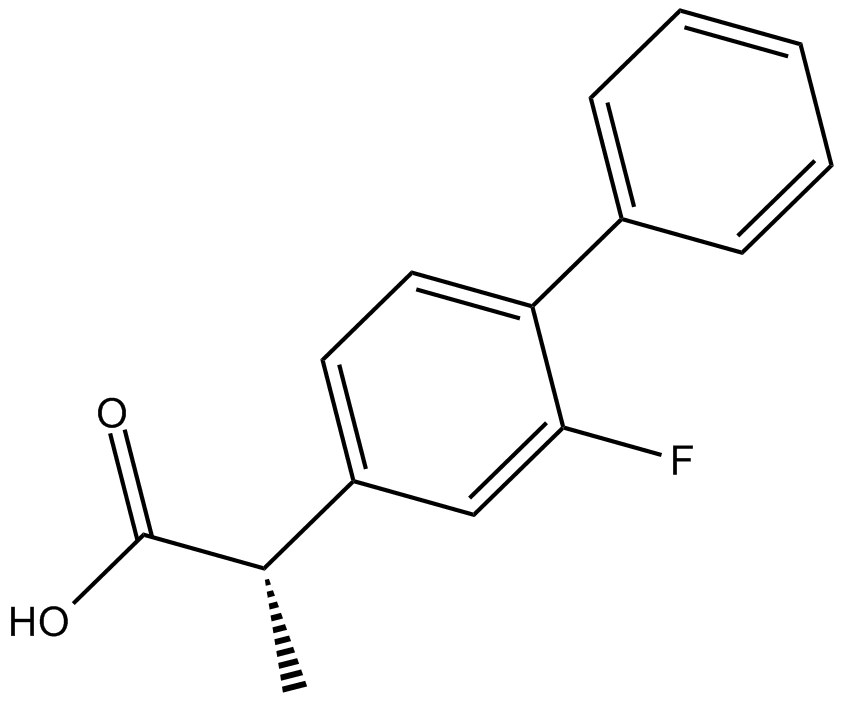
Cas No.: 51543-39-6
Sample solution is provided at 25 µL, 10mM.
IC50: 0.5 μM for both COX-1 and COX-2 in guinea pig whole blood
(S)-Flurbiprofen is a dual inhibitor of COX-1 and COX-2.
Non-steroidal anti-inflammatory drugs (NSAIDs) are potent inhibitors of both COX-1 and COX-2, but are frequently supplied as racemates. In the 2-aryl propionic acid NSAID family, cyclooxygenase inhibition resides primarily in the (S)-enantiomer.
In vitro: It was found that the IC50 value of (S)-flurbiprofen was about 0.5 μM for both COX-1 and COX-2 in guinea pig whole blood [1].
In vivo: The efficacy of multiple applications of S(+)-flurbiprofen plaster (SFPP) was evaluated for the alleviation of inflammatory pain and edema in rat adjuvant-induced arthritis model. Multiple applications of SFPP could exert a significant analgesic effect from the first day of application as compared to the other NSAID drugs. In terms of paw edema, SFPP could decrease edema from the second day after application. Moreover, multiple applications of SFPP were found to be superior to those of other NSAID drugs in terms of the analgesic effect [2].
Clinical trial: Previous clinical study showed that 40 mg of S-flurbiprofen plaster showed remarkable pain relief in not only primary endpoint but also all the other endpoint with significant differences over placebo. The safety profile of S-flurbiprofen plaster 40 mg was not different from that of placebo. Thus, 40 mg was determined as the optimal tested dose [3].
References:
[1] Barnett, J. ,Chow, J.,Ives, D., et al. Purification, characterization and selective inhibition of human prostaglandin G/H synthase 1 and 2 expressed in the baculovirus system. Biochimica et Biophysica Acta 1209, 130-139 (1994).
[2] Sugimoto M et al. Topical Anti-Inflammatory and Analgesic Effects of Multiple Applications of S(+)-Flurbiprofen Plaster (SFPP) in a Rat Adjuvant-Induced Arthritis Model. Drug Dev Res. 2016 Jun;77(4):206-11.
[3] Yataba I, Otsuka N, Matsushita I, Matsumoto H, Hoshino Y. The efficacy and safety of S-flurbiprofen plaster in the treatment of knee osteoarthritis: a phase II, randomized, double-blind, placebo-controlled, dose-finding study. J Pain Res. 2017 Apr 11;10:867-880.
Average Rating: 5 (Based on Reviews and 40 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















