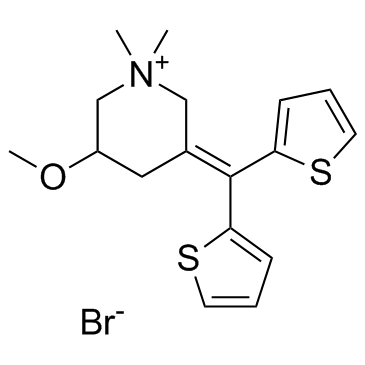
Timepidium bromide (Sesden) |
| Catalog No.GC32666 |
Le bromure de timepidium (Sesden) (Sesden; SA504) est un agent anticholinergique.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 35035-05-3
Sample solution is provided at 25 µL, 10mM.
Timepidium bromide (Sesden; SA504) is an anticholinergic agent.
Effects of Timepidium bromide (TB), acetylcholine (ACh) and neostigmine (Neost) on gastric and duodenal blood flow distribution are studied by the use of 131I-labeled macroaggregated human serum albumin (MAA) in rabbits. In normal rabbits, gastric blood flow is found to be uneven in various regions of the stomach: anterior corpus (50% of total gastric blood flow) greater than posterior corpus (40%) greater than pyloric antrum (7%). Intravenous administration of Timepidium bromide (200 μg/kg) to normal rabbits produces a slight increase in total gastric blood flow, but the increase in the mucosal layer of the pyloric antrum is considerable[1].
[1]. Naito K, et al. Effect of timepidium bromide, an anticholinergic agent, on gastric and duodenal blood flow distribution in rabbits. Jpn J Pharmacol. 1982 Feb;32(1):73-80.
Average Rating: 5 (Based on Reviews and 15 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















