Tivozanib (AV-951) |
| Catalog No.GC12036 |
Le tivozanib (AV-951 ; KRN951) est un inhibiteur de la tyrosine kinase VEGFR puissant, sélectif et actif par voie orale avec une IC50 de 0,21, 0,16 et 0,24 nM pour VEGFR-1, VEGFR-2, VEGFR-3, respectivement. Le tivozanib inhibe l'angiogenèse et la perméabilité vasculaire dans les tissus tumoraux et présente une activité antitumorale. Le tivozanib a le potentiel pour la recherche sur le carcinome À cellules rénales métastatique (RCC) .
Products are for research use only. Not for human use. We do not sell to patients.
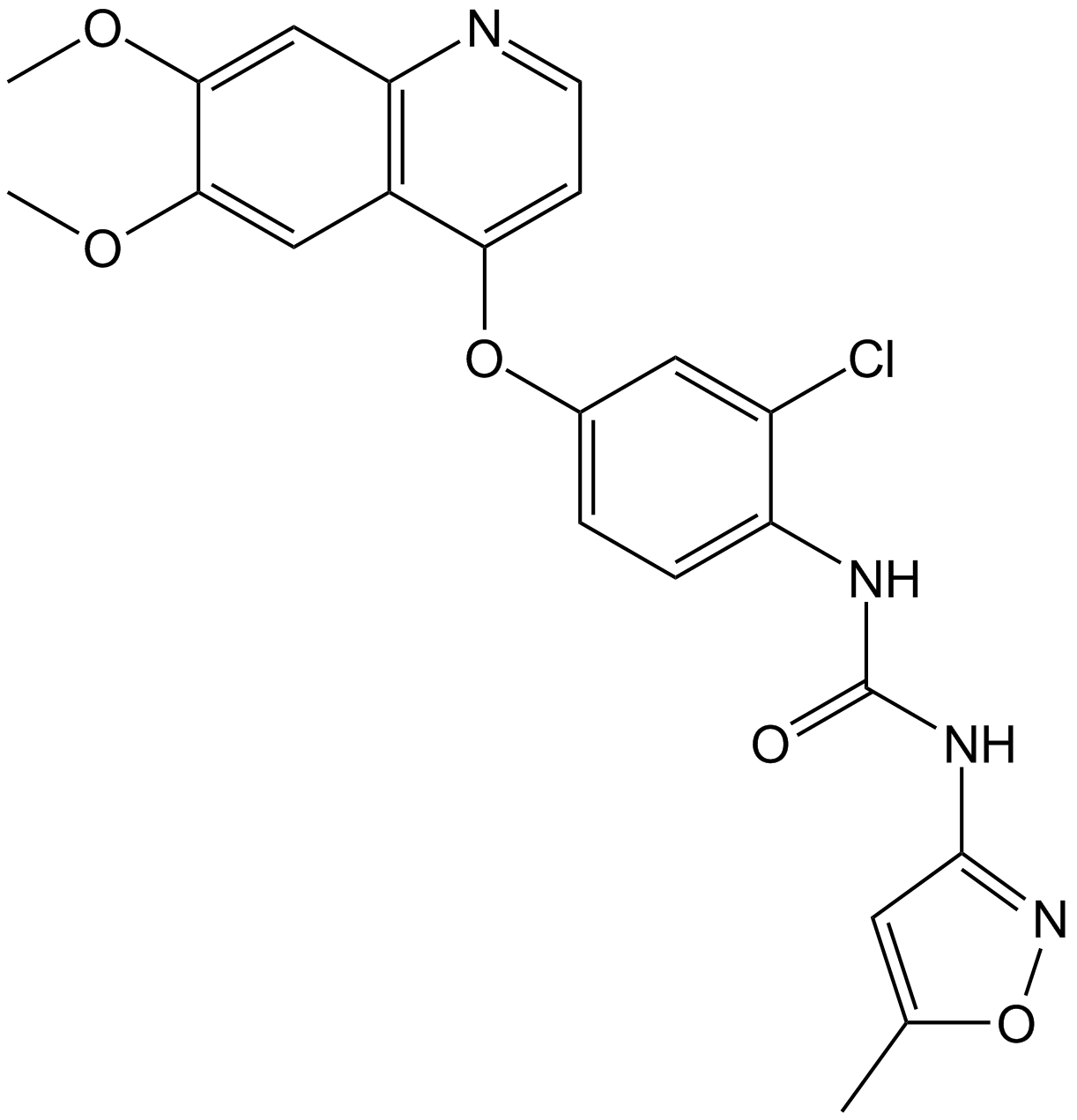
Cas No.: 475108-18-0
Sample solution is provided at 25 µL, 10mM.
Average Rating: 5 (Based on Reviews and 32 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















