TL-895 |
| Catalog No.GC63229 |
Le TL-895 est un inhibiteur de BTK irréversible puissant, actif par voie orale, compétitif pour l'ATP et hautement sélectif avec une IC50 et un Ki de 1,5 nM et 11,9 nM, respectivement .
Products are for research use only. Not for human use. We do not sell to patients.
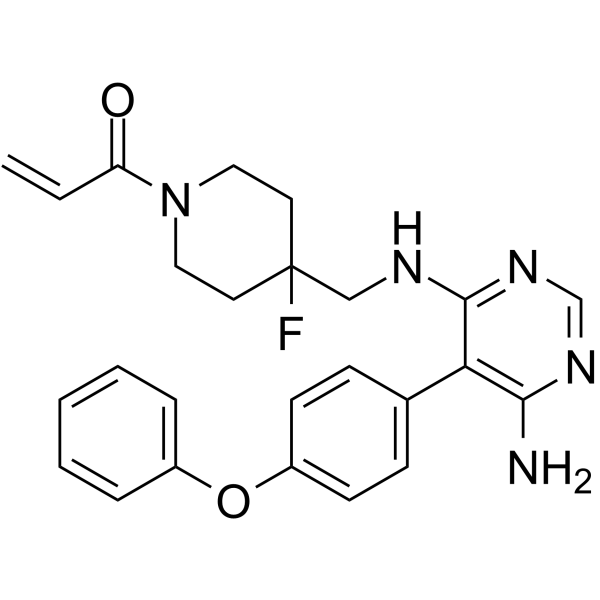
Cas No.: 1415823-49-2
Sample solution is provided at 25 µL, 10mM.
TL-895 is a potent, orally active, ATP-competitive, and highly selective irreversible BTK inhibitor with an IC50 and a Ki of 1.5 nM and 11.9 nM, respectively[1]. TL-895 is used be for JAKi-relapsed/refractory myelofibrosis, acute myeloid leukemia, COVID-19 and cancer research[2][3][4].
[1]. Richard D Caldwell, et al. Discovery of Evobrutinib: An Oral, Potent, and Highly Selective, Covalent Bruton’s Tyrosine Kinase (BTK) Inhibitor for the Treatment of Immunological Diseases. J Med Chem. 2019 Sep 12;62(17):7643-7655.
[2]. Valeria Di Battista, et al. Genetics and Pathogenetic Role of Inflammasomes in Philadelphia Negative Chronic Myeloproliferative Neoplasms: A Narrative Review. Int J Mol Sci
[3]. A Study of TL-895 With Standard Available Treatment Versus Standard Available Treatment for the Treatment of COVID-19 in Patients With Cancer
[4]. Study of TL-895 in Subjects With Myelofibrosis
Average Rating: 5 (Based on Reviews and 5 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















