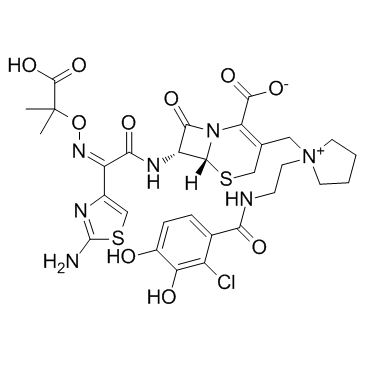
Cefiderocol (S-649266)
Cefiderocol is an antibiotic that is prescribed to treat severe urinary tract infections when no other treatment alternatives are available. It is marketed under the trade name Fetroja among others.Pseudomonas aeruginosa and other multidrug-resistant Gram-negative bacteria can be treated with it. It is administered by venous injection. Its structure is comparable to that of cefepime and ceftazidime, but it is a siderophore because of the chlorocatechol group at the end of the C-3 side chain, which further increases its β-lactamase stability. Cefiderocol exhibits a broad spectrum of action against all types of carbapenemases, including metallo-β-lactamases (class B such as VIM, NDM, and IMP) and serine-carbapenemases (class A such as KPC and class D such as OXA-48). Cefiderocol also demonstrates good stability against β-lactamases. The chemical strucure of Cefiderocol is shown in Figure 1.
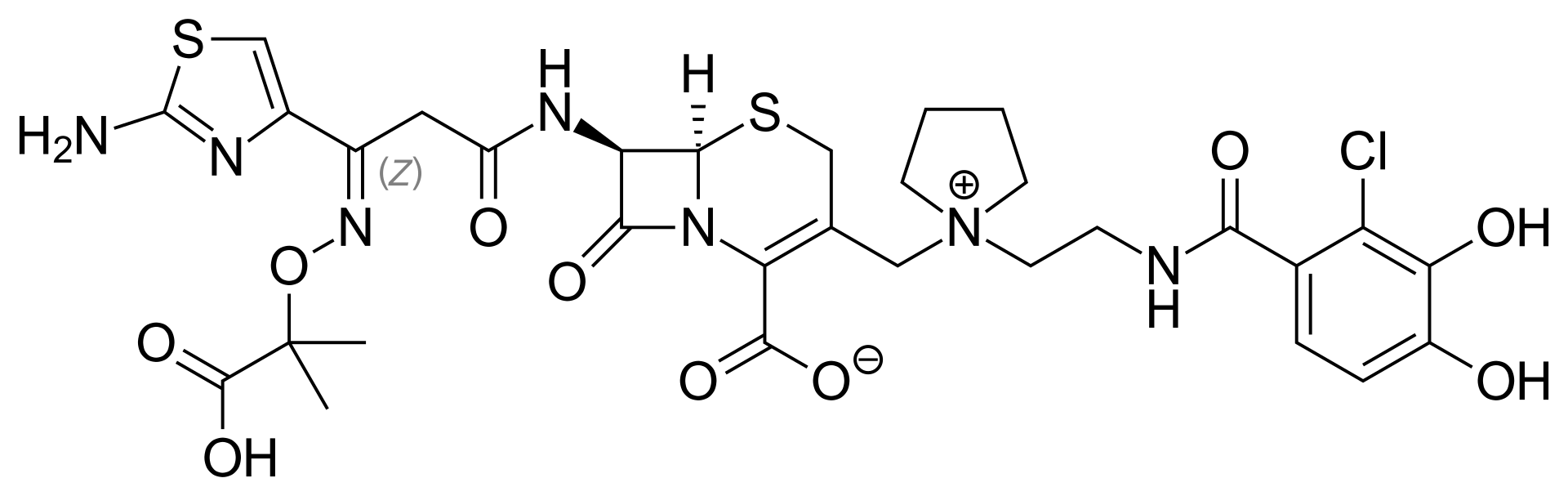
Figure 1: The molecular structure of Cefiderocol.
Aerobic, Gram-negative bacterial infections, specifically carbapenem-resistant Enterobacteriaceae, multidrug-resistant Pseudomonas aeruginosa, and multidrug-resistant Acinetobacter baumannii, are being developed as treatments with cefiderocol . Cefiderocol is intended to be administered intravenously. Despite decades of research, no siderophore antibiotic is currently approved for use in clinical settings to treat bacterial infections. Cefiderocol is an innovative injectable siderophore cephem antibiotic that possesses a catechol moiety. This allows it to form a chelating complex with ferric iron and effectively penetrate the outer cell wall of bacteria by utilizing their iron transport mechanism. The idea of introducing substances into other cells, like bacteria, has been compared to the Trojan Horse. Cefiderocol demonstrates a strong affinity for penicillin-binding proteins, particularly Enterobacteriaceae's penicillin-binding protein 3 and nonfermenting bacteria, upon entry into the Gram-negative periplasmic region. This leads to the inhibition of bacterial growth through the suppression of bacterial cell wall formation. Comparing cefiderocol to commercial medicines like meropenem, the kinetic tests employing isolated carbapenemases such imipenemase-1, Verona integron-encoded metallo-β-lactamase-2 L-1, and oxacillinase-23 revealed that cefiderocol has low kcat/Km values against all of these carbapenemases. These findings show that cefiderocol exhibits good stability against metallo-β-lactamase (class B) as well as serine-type β-lactamases, such as class D.
Cefiderocol accounted for 90.6% of the administered dose discharged into urine and 92.3% of the area under the concentration-time curve of total radioactivity in plasma, according to the results of metabolite profiling. Figure 2 displays the concentration-time profiles of cefiderocol in plasma and total radioactivity in whole blood and plasma following the delivery of [14C]-cefiderocol. When compared to analogous values in whole blood, the general mean (GM) of the Cmax and AUC0-inf for total radioactivity in plasma was about two times greater. The GM of the t1/2,z for total radioactivity was similar in both matrices. The general mean of the Cmax and AUC0-inf of cefiderocol in plasma matched the corresponding total radioactivity measurements. The general mean of the t1/2,z values in plasma was similar for total radioactivity and cefiderocol. In comparison to plasma total radioactivity, the GM of the CL and Vz values for plasma cefiderocol were almost two times lower than those for whole blood total radioactivity. With the exception of Vz, the statistical analysis showed that there were no statistically significant differences in PK parameters between cefiderocol and total radioactivity in plasma. Furthermore, the statistical analysis showed that there were statistical differences between the total radioactivity in whole blood and the total radioactivity in plasma or cefiderocol in plasma in terms of Cmax, AUC0-last, AUC0-inf, CL, and Vz.
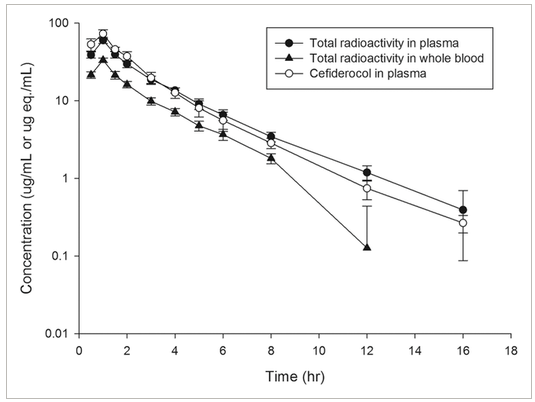
Figure 2: Concentration-time profiles of total radioactivity in whole blood and plasma and cefiderocol in plasma following a single intravenous infusion of 1000 mg of [14C]-cefiderocol over 1 hour.
Six participants had their urine and feces collected 120 hours after [14C]-cefiderocol was started on an infusion. Between 0 and 6 hours and 0 to 48 hours, the mean urine excretion of radioactivity was roughly 74.6% and 98.5%, respectively. There was very little radioactive excretion in the stool on average. Over the course of 120 hours following dosage, the mean total recovery of radioactivity in urine and feces was 101.5% of the dose that was given. Representative radio-chromatograms for pooled plasma, urine, and feces are shown in Figure 3.
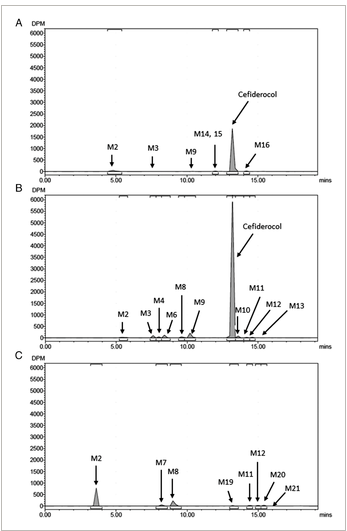
Figure 3: Representative radio-chromatograms of (A) 0-16-hour pooled plasma extracts, (B) 0-48-hour pooled urine, (C) 0-72-hour pooled feces extracts following a single intravenous infusion of 1000 mg of [14C]-cefiderocol over 1 hour.
Cefiderocol was the main peak on the radio-chromatograms of the plasma extracts in all six participants, and it accounted for 92.3% of the total radioactivity for the plasma AUC as a mean value. PCBA (M2), M3, M9, M14, M15, and M16 were among the minor peaks that were noted. Cefiderocol was the main peak on the urine radio-chromatograms for all six participants, and its mean value represented 90.6% of the dose that was given. Cefiderocol-7-epi (M10), cefiderocol catechol 3-methoxy (M11), cefiderocol catechol 4-methoxy (M12) and M13, and PCBA (M2) were among the minor peaks that were noticed. PCBA (M2), M7, M8; cefiderocol catechol 3-methoxy (M11); and cefiderocol catechol 4-methoxy (M12), M19, M20, and M21 were detected as minor peaks on the radio-chromatograms of the fecal samples. At m/z 751.8, the peak that is thought to be cefiderocol has a protonated molecule. The retention duration of this peak is roughly 13.4 minutes. The product ion spectrum and retention time were in agreement with the cefiderocol reference standard.
Metabolites M2, M10, M11, and M12 exhibited a protonated molecule at m/z 285, m/z 751.8, m/z 383.7 (as double-charged ion), and m/z 383.7 (as double-charged ion), respectively, with retention times of roughly 6.0, 13.8, 14.4, and 14.8 minutes. Both the product ion spectrum and retention time were in agreement with the reference standards. PCBA, cefiderocol-7-epi, cefiderocol catechol 4-methoxy, and cefiderocol catechol 3-methoxy were found to be the metabolites M2, M10, M11, and M12. The protonated molecule of Metabolite M1, which had a retention duration of about 5.5 minutes, was located at m/z 461, 176 Da higher than that of PCBA (m/z 285). The loss of the glucuronic acid moiety (176 Da) resulted in the formation of a base peak at m/z 285 in the product ion spectra of metabolite M1. It was suggested that metabolite M1 was a PCBA compound of glucuronic acid.The protonated molecule of Metabolite M4, which had a retention duration of about 8.2 minutes, was located at m/z 365, which is 80 Da higher than that of PCBA (m/z 285). The loss of the sulfonic acid moiety (80 Da) resulted in the base peak seen in the product ion spectra of M4. It was proposed that metabolite M4 was PCBA sulfate.
Cefiderocol's actual mean Cmax and AUC values matched those of total radioactivity. According to the statistical study, cefiderocol and total radioactivity in plasma had comparable PK characteristics. These findings suggest that cefiderocol accounted for the majority of the total radioactivity in plasma. Cefiderocol's outcome is comparable to that of cefepime, another commercially available aminothiazolyl oximino cephalosporin . The total radioactivity AUC ratio of whole blood to plasma was similar to the hematocrit values, indicating that there was no partitioning of total radioactivity into RBCs. The association of total radioactivity into RBCs was established, and mean partitioning values showed less than zero at the calculable time points. The reason why the total radioactivity in plasma was almost two times higher than that in whole blood can be attributed to the lack of any partitioning of total radioactivity into RBCs.
It has been proposed that intact cefiderocol is linked to the radioactivity found in plasma; cefiderocol accounted for more than 92% of the plasma's AUC of total radioactivity. There was very little radioactivity divided among RBCs overall. Ninety-nine percent of the injected dose of radioactivity was eliminated in urine, with very little radioactivity excreted in the feces. According to this study, the body did not retain cefiderocol or drug-related substances. Cefiderocol was mainly eliminated in urine as an unaltered medication (>90% of the dose that was given). Cefiderocol's metabolic pathways were secondary routes of elimination. In healthy adult male participants, intravenous injection of a single 1000-mg (∼100-μCi) dosage of [14C]-cefiderocol over an hour was found to be generally safe and well tolerated.













Commentaires