RIPA Lysis Buffer (Strong)
RIPA Lysis Buffer is a traditional rapid lysis buffer for cellular tissues. It is a lysis and washing buffer that is used in reporter gene detection, protein kinase experiments, immunoassays, and protein purification. The main components of RIPA Lysis Buffer (Strong) are 50 mMTris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS and general protease and phosphatase inhibitors (such as sodium orthovanadate, Sodium fluoride and EDTA).Store at -20°C 12 months. This product can be used for cell or tissue samples of animals and plants, as well as fungal or bacterial samples.
The molecular weight of RIPA (Radio immunoprecipitation assay) lysis buffer, including a “strong” formulation, cannot be directly determined because RIPA buffer is a mixture of several components, each with its own molecular weight. Here are the approximate molecular weights of the common components:
Tris-HCl: ~121.1 g/mol
NaCl: ~58.44 g/mol
Triton X-100: ~647.9 g/mol
Sodium deoxycholate: ~430.6 g/mol
SDS (Sodium dodecyl sulfate): ~288.4 g/mol
Keep in mind that the exact composition of a “strong” RIPA lysis buffer can vary depending on the specific protocol or recipe used by researchers.
It is Supplied as a ready to use solution that requires no preparation.
The amount of lysis buffer should be empirically determined for each cell type to ensure efficient lysis . Add 10 to 100 µl of RIPA Lysis Buffer with Inhibitors per 1 x 106 cells.
This buffer is more denaturing than NP-40 or Triton X-100 because it contains the ionic detergents SDS and sodium deoxycholate as active constituents and is particularly useful for disruption of nuclear membranes in the preparation of nuclear extracts. The stronger detergents in RIPA buffer (such as SDS) cause greater protein denaturation and decrease protein-protein interactions. It is used for tissue extraction because it’s a harsher buffer and it can solubilize nuclear membranes. Because of this it is used for Sample preparations for proteomics of breast cancer.
The procedure of RIPA( Radio immunoprecipitation Assay) lysis buffer involves a combination of elements that work synergistically to interact with cellular proteins .
Let’s break down the mechanism of action of the key components in the RIPA lysis buffer:
Tris–HCl (pH 7.4): Provides a buffering system to maintain a stable pH during the lysis process.
NaCl (150 mM): Provides isotonic conditions and helps in maintaining cell morphology during lysis.
Triton X-100 (1%): A non-ionic detergent that solubilizes cell membranes by disrupting lipid-protein interactions, contributing to cell lysis.
Sodium deoxycholate (1%): An ionic detergent that further aids in solubilizing membranes by disrupting protein-protein interactions.
SDS (0.1%): A strong anionic detergent that denatures proteins by disrupting their secondary and tertiary structures. This contributes to the overall denaturing capabilities of the buffer.
EDTA : A chelating agent that sequesters divalent cations and helps prevent enzymatic degradation of proteins.
PMSF : A serine protease inhibitor that helps to inhibit proteases and prevent protein degradation during cell lysis.
Sodium orthovanadate : An inhibitor of tyrosine phosphatases, which helps preserve phosphorylated proteins by inhibiting their DE phosphorylation.
Protease inhibitor cocktail : Contains a mixture of protease inhibitors to inhibit a broad spectrum of proteases, further preventing protein degradation during lysis.
The mechanism of action involves these components working together to disrupt cell membranes, solubilize proteins, and inhibit protease activity, ultimately allowing the extraction of proteins for downstream analysis.
Versatility of RIPA lysis Buffer is further augmented by its compatibility with protease and phosphatase inhibitors, stability, and ability to minimize non-specific protein-binding interactions leading to low backgrounds in immunoprecipitation. Still, RIPA buffer is very compatible with a myriad of applications, including reporter assays, protein assays, immunoassays and protein purification.
When protein quantitation is desired, RIPA buffer is the lysis buffer of choice due to its compatibility with the BCA Protein Assay, although it can denature kinases and can disrupt protein-protein interactions in immunoprecipitation/pull down assays.
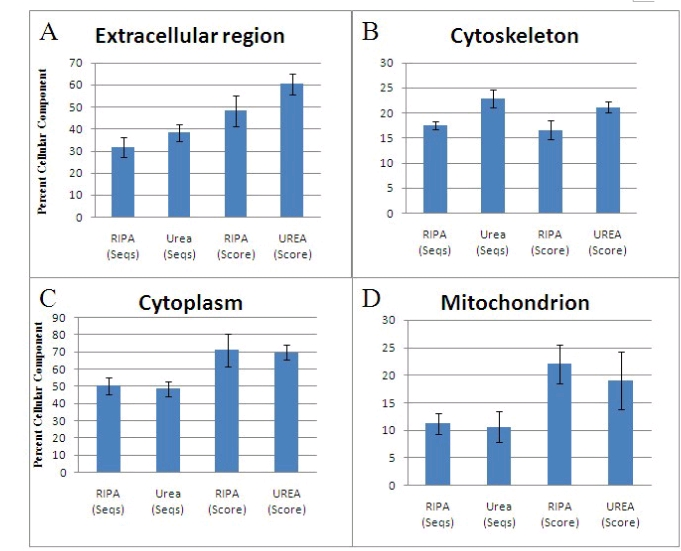
Figure 1: shows that proteins of the extracellular region (A) and cytoskeleton (B) are more soluble in urea buffer than in RIPA, whereas for cytoplasmic (C) and mitochondrial (D) proteins, RIPA buffer is preferred. (NCBI).
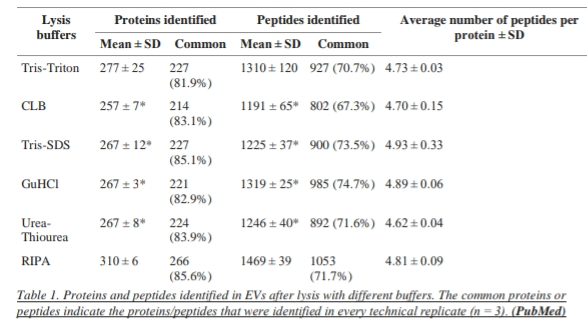
It is suggested that RIPA is an optimal lysis buffer for extracellular vesicles in combination with MS.RIPA buffer is compared with other buffers (Tris-SDS, Tris-Triton, GuHCL, urea-thiourea, and commercial cell lysis buffer) and RIPA buffer outperformed the other buffers investigated in this study. Following lysis with RIPA Buffer 310 proteins and 1469 peptides were identified using LTQ OrbitrapXL mass Spectrometer. In the case of other buffers. Tris-Triton buffer identifies 277 proteins, cell lysis buffer identifies 257 proteins and Tris-SDS, GuHCL, and urea thiourea each identify 267 proteins. In total 399 proteins including 74 of the top EV markers were identified, the most of the latter using RIPA.
The proteins exclusively identified using RIPA represented all Gene Ontology cell compartments.
It was identified by the research work and then the analysis of the research that highest number of proteins (310 ± 6) and peptides (1469 ± 39) were identified after lysis with RIPA buffer in comparison to the other Buffers so we can say that for lysis of extracellular vesicles RIPA lysis buffer is preferred because of its efficiency.
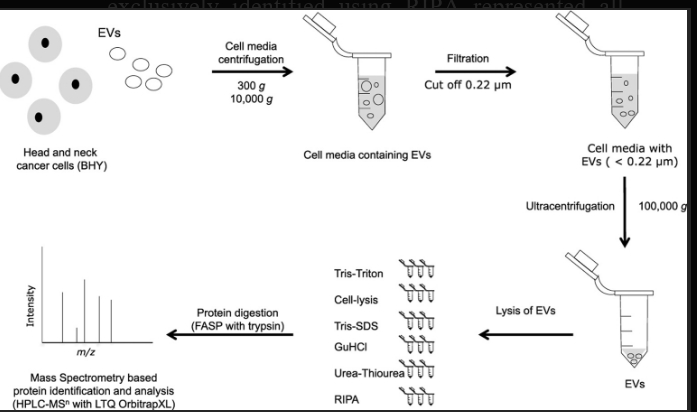
Figure 2: shows that RIPA Lysis Buffer used to isolate proteins from extracellular vesicles for mass Spectrometry-based proteomic analysis.
The highest number of proteins (310 ± 6) and peptides (1469 ± 39) were Identified after lysis with RIPA buffer. There was no significant difference in the average number of peptides per protein between RIPA and the other buffers. In terms of repeatability, 86% of proteins and 72% of peptides were identified in all three replicates when the EVs were lysed with RIPA buffer.
RIPA buffer’s denaturing properties, attributed to detergents like SDS, make it effective for extracting nuclear and mitochondrial proteins. However, its denaturing effects may not be ideal for preserving protein-protein interactions compared to milder buffers like NP-40 or Triton X-100.
The pH of RIPA buffer can significantly impact its protein solubilization capacity. The pH influences the effectiveness of solubilizing membrane proteins, with advanced pH situations enhancing solubilization. conforming the pH of the buffer can help overcome pH-dependent goods and ameliorate the birth of proteins, especially membrane proteins. It’s essential to optimize the pH conditions grounded on the specific proteins of interest to achieve optimal solubilization and birth effectiveness.
Though different variations of RIPA buffer have been used, protein extraction usingRIPA buffer usually generates two distinctive fractions: RIPA-soluble and RIPA-insoluble fractions. Generally, only RIPA-soluble fraction is used for downstream experiments. RIPA-insoluble fraction is discarded. It is found that certain proteins are more soluble in RIPA buffer than others. Fibronectin From F9 aggregated was found poorly solubilized in RIPA buffer and equal amount of Septin . From mouse front cortex sample was found in both RIPA-soluble and RIPA-insoluble fractions indicating that the efficiency of protein extraction by RIPA buffer is low for these particular proteins.The choice of lysis buffer should be grounded on the specific characteristics of the proteins being studied to insure optimal birth effectiveness.Cell lysate from RIPA buffer extraction was found to artificially increase the activity of Certain protein kinases. The in vitro protein kinase activity derived from RIPA buffer lysate of colon Cancer cells was found to be elevated five-seven folds as compared to the activity from the same cells using NP-40 as cell lysis reagent.RIPA Lysis Buffer is designed for use in laboratory settings and is not intended for direct application on or ingestion by humans. Its impact is indirect, aiding researchers in understanding cellular processes for advancements in various fields.














Commentaires