Mefenamic Acid (Synonyms: C.I. 473, CN 35355, NSC 94437) |
| Catalog No.GC11096 |
L'acide méfénamique est un agent anti-inflammatoire non stéroÏdien, agissant comme un inhibiteur compétitif de hCOX-1 et hCOX-2, avec des IC50 de 40 nM et 3 μM pour hCOX-1 et hCOX-2, respectivement.
Products are for research use only. Not for human use. We do not sell to patients.
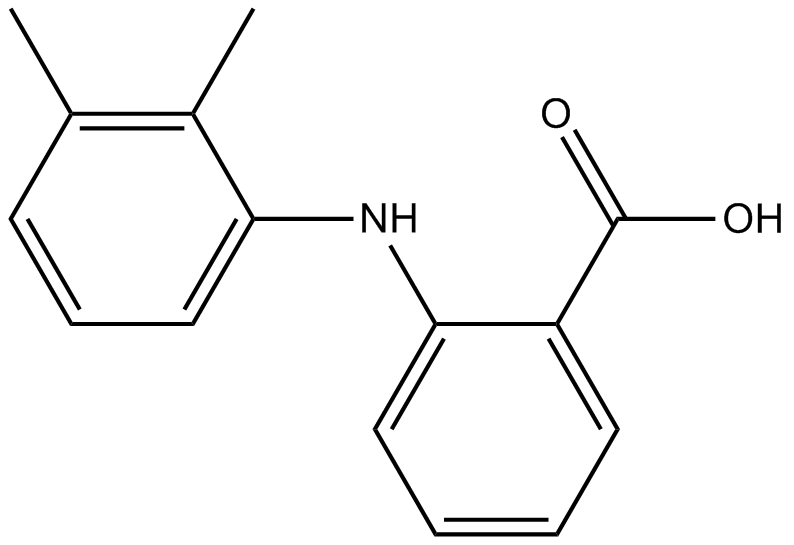
Cas No.: 61-68-7
Sample solution is provided at 25 µL, 10mM.
Mefenamic acid is a non-steroidal anti-inflammatory agent, acting as a competitive inhibitor of hCOX-1 and hCOX-2, with IC50s of 40 nM and 3 μM for hCOX-1 and hCOX-2, respectively.
Mefenamic acid is a non-steroidal anti-inflammatory agent, acting as a competitive inhibitor of hCOX-1, with IC50s of 40 nM and 3 μM for hCOX-1 and hCOX-2, respectively[1]. Mefenamic acid (0-100 μM) has cytotoxic effects on KB, Saos-2, and 1321N cells, however, U-87MG cells are resistant to Mefenamic acid[2].
References:
[1]. Gierse JK, et al. Expression and selective inhibition of the constitutive and inducible forms of human cyclo-oxygenase. Biochem J. 1995 Jan 15;305 (Pt 2):479-84.
[2]. Hashemipour MA, et al. In Vitro Cytotoxic Effects of Celecoxib, Mefenamic Acid, Aspirin and Indometacin on Several Cells Lines. J Dent (Shiraz). 2016 Sep;17(3):219-25.
Average Rating: 5 (Based on Reviews and 36 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















