Desonide (Synonyms: Budesonide acetonide) |
| Catalog No.GC15690 |
Le désonide est un anti-inflammatoire corticostéroÏde non fluoré utilisé par voie topique pour les dermatoses.
Products are for research use only. Not for human use. We do not sell to patients.
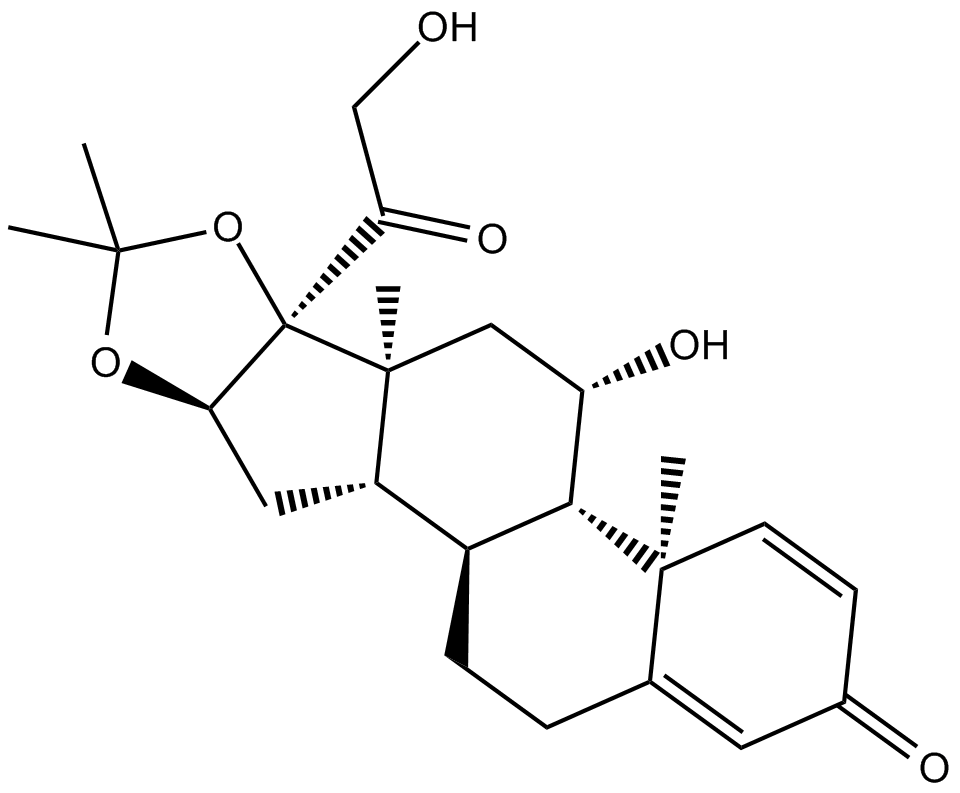
Cas No.: 638-94-8
Sample solution is provided at 25 µL, 10mM.
Desonide is a nonfluorinated corticosteroid anti-inflammatory agent used topically for dermatoses.Target: Glucocorticoid ReceptorDesonide is a low-potency topical corticosteroid that has been used for decades in the treatment of steroid-responsive dermatoses [1]. Desonide induced significant colorimetric improvement compared with placebo. A good to excellent response was achieved in 30% for desonide, and 6% for placebo. Decreased pigmentation in the desonide-treated axillae was associated with recovery of disruption at the basal membrane. Desonide showed depigmenting properties in women with axillary hyperpigmentation [2]. Given the favorable safety profile of all other desonide preparations and their utility as a low potency corticosteroid, desonide foam promises to be a useful addition to the armamentarium, when other desonide vehicles might be less acceptable [3].
References:
[1]. Kahanek, N., C. Gelbard, and A. Hebert, Desonide: a review of formulations, efficacy and safety. Expert Opin Investig Drugs, 2008. 17(7): p. 1097-104.
[2]. Castanedo-Cazares, J.P., et al., Topical niacinamide 4% and desonide 0.05% for treatment of axillary hyperpigmentation: a randomized, double-blind, placebo-controlled study. Clin Cosmet Investig Dermatol, 2013. 6: p. 29-36.
[3]. Parish, D. and N. Scheinfeld, Desonide foam: a review. Drugs Today (Barc), 2008. 44(1): p. 55-62.
Average Rating: 5 (Based on Reviews and 40 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















