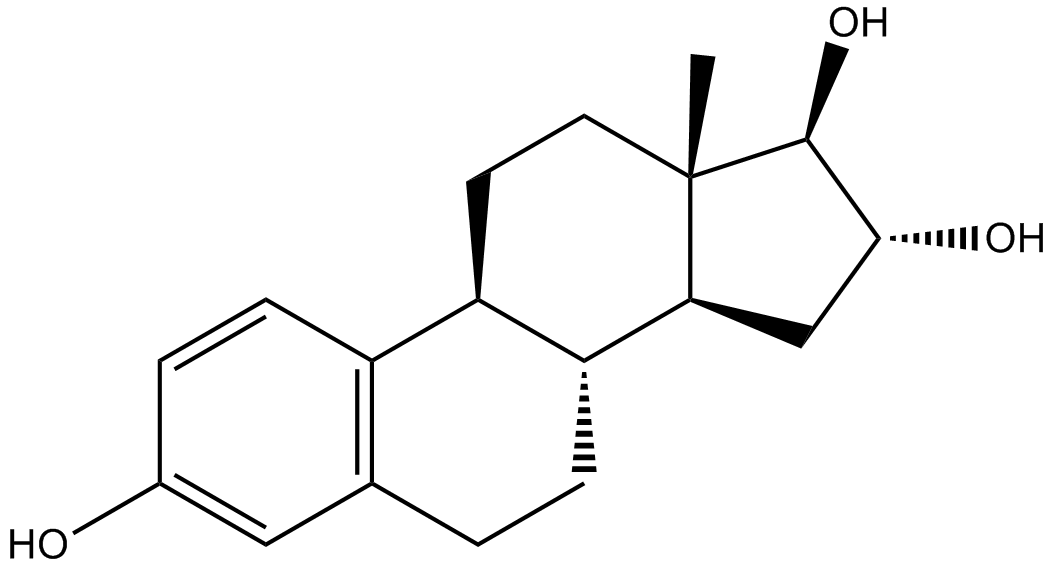
Estriol (Synonyms: E3, 16α-hydroxy-17β-Estradiol, 1,3,5(10)-Estratriene-3,16α,17β-triol) |
| Catalog No.GC12182 |
L'œstriol est un antagoniste du récepteur des œstrogènes couplé À la protéine G dans les cellules cancéreuses du sein négatives pour les récepteurs des œstrogènes.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 50-27-1
Sample solution is provided at 25 µL, 10mM.
Estriol is an antagonist of the G-protein coupled estrogen receptor in estrogen receptor-negative breast cancer cells.Target: Estrogen Receptor/ERRA recent study shows that estrogen (estrone, estradiol, and estriol) inhibits Alzheimer's disease-associated low-order Aβ oligomer formation, and among them, estriol shows the strongest in vitro activity [1]. In mPTEN+/- mice, estriol treatments resulted in a 187.54% gain in the relative ratio of uterine wet weight to body weight; estriol also increases the ratio to 176.88% in wild-type mice [2]. Estriol treatment (20 mg/kg ip), in vivo, sensitizes Kupffer cells to LPS via mechanisms dependent on an increase in CD14 by elevated portal blood endotoxin caused by increased gut permeability in rats; while one-half of the rats given estriol intraperitoneally 24 hours before an injection of a sublethal dose of LPS (5 mg/kg) died within 24 hours [3].
References:
[1]. Morinaga, A., et al., Effects of sex hormones on Alzheimer's disease-associated beta-amyloid oligomer formation in vitro. Exp Neurol, 2011. 228(2): p. 298-302.
[2]. Begum, M., et al., Neonatal estrogenic exposure suppresses PTEN-related endometrial carcinogenesis in recombinant mice. Lab Invest, 2006. 86(3): p. 286-96.
[3]. Hewitt, S.C. and K.S. Korach, Estrogenic activity of bisphenol A and 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE) demonstrated in mouse uterine gene profiles. Environ Health Perspect, 2011. 119(1): p. 63-70.
Average Rating: 5 (Based on Reviews and 4 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















