Risdiplam (RG7916) (Synonyms: RG-7916, RO7034067) |
| Catalog No.GC30845 |
Risdiplam (RG7916) (RG7916) est un modificateur d'épissage du pré-ARNm SMN2 administré par voie orale, distribué au centre et À la périphérie, qui augmente les niveaux de protéine du neurone moteur de survie (SMN).
Products are for research use only. Not for human use. We do not sell to patients.
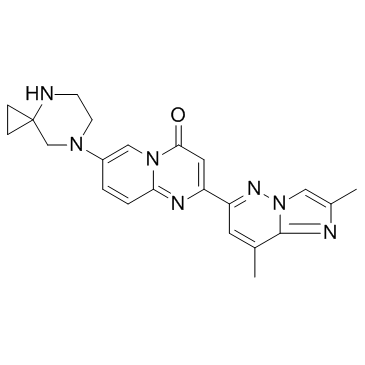
Cas No.: 1825352-65-5
Sample solution is provided at 25 µL, 10mM.
Risdiplam is a small molecule SMN2 pre-mRNA splicing modifier that promotes the inclusion of exon 7 and production of full-length SMN2 mRNA, which can compensate for the loss of SMN1 [1-2].
Risdiplam (RG7916)(0-1µM) was active in vitro in SMA patient-derived fibroblasts and in motor neurons generated from induced pluripotent stem cells (iPSCs) derived from SMA type 1 patient fibroblasts, promoting the inclusion of exon 7, to generate full-length (FL) mRNA[1].
Risdiplam (RG7916) potently increases SMN protein in both brain and muscle tissues of transgenic mouse models of SMA[1]. In infants with type 1 spinal muscular atrophy, treatment with oral risdiplam(0.08-0.2mg/kg/day)led to an increased expression of functional SMN protein in the blood[3].
References:
[1]. Ratni H, Ebeling M,et,al. Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 ( SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA). J Med Chem. 2018 Aug 9;61(15):6501-6517. doi: 10.1021/acs.jmedchem.8b00741. Epub 2018 Jul 25. PMID: 30044619.
[2]. Singh RN, Ottesen EW, et,al. The First Orally Deliverable Small Molecule for the Treatment of Spinal Muscular Atrophy. Neurosci Insights. 2020 Nov 23;15:2633105520973985. doi: 10.1177/2633105520973985. PMID: 33283185; PMCID: PMC7691903.
[3]. Baranello G, Darras BT, et,al. FIREFISH Working Group. Risdiplam in Type 1 Spinal Muscular Atrophy. N Engl J Med. 2021 Mar 11;384(10):915-923. doi: 10.1056/NEJMoa2009965. Epub 2021 Feb 24. PMID: 33626251.
Average Rating: 5 (Based on Reviews and 21 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















