Rivastigmine Tartrate (Synonyms: Exelon, SDZENA 713) |
| Catalog No.GC12994 |
Le tartrate de rivastigmine (ENA 713; SDZ-ENA 713) est un puissant inhibiteur de la cholinestérase (ChE) actif par voie orale et inhibe la butyrylcholinestérase (BChE) et les acétylcholinestérases (AChE) avec des IC50 de 0,037μM, 4,15μM, respectivement.
Products are for research use only. Not for human use. We do not sell to patients.
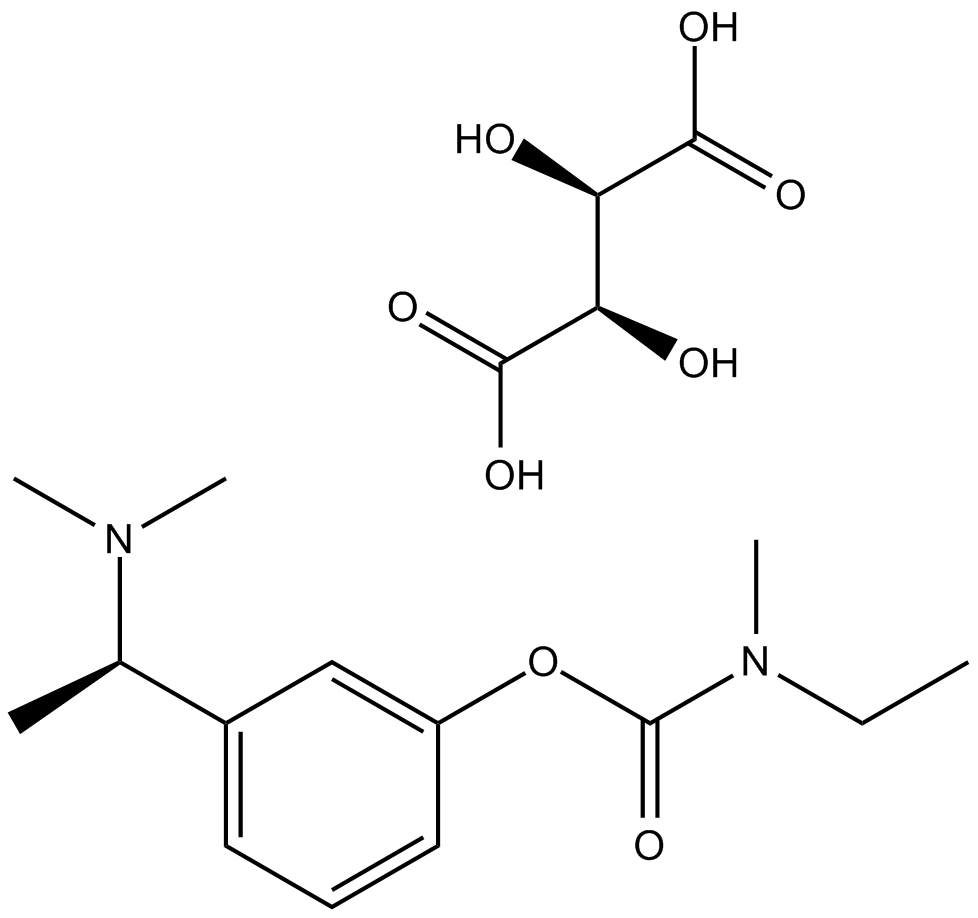
Cas No.: 129101-54-8
Sample solution is provided at 25 µL, 10mM.
Rivastigmine tartrate, an cholinesterase inhibitor(IC50= 5.5 uM), inhibits both butyrylcholinesterase and acetylcholinesteraseIC50 value: 5.5 uMTarget: AChERivastigmine is a parasympathomimetic or cholinergic agent for the treatment of mild to moderate dementia of the Alzheimer's type and dementia due to Parkinson's disease. The drug can be administered orally or via a transdermal patch; the latter form reduces the prevalence of side effects, which typically include nausea and vomiting. The drug is eliminated through the urine, and appears to have relatively few drug-drug interactions. Rivastigmine, a cholinesterase inhibitor, inhibits both butyrylcholinesterase and acetylcholinesterase. It is thought to work by inhibiting these cholinesterase enzymes, which would otherwise break down the brain chemical acetylcholine.
References:
[1]. Kurz A, Farlow M, Lefèvre G. Pharmacokinetics of a novel transdermal rivastigmine patch for the treatment of Alzheimer's disease: a review. Int J Clin Pract. 2009 May;63(5):799-805.
[2]. Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer's disease. Clin Ther. 1998 Jul-Aug;20(4):634-47.
[3]. Stryjer R, Ophir D, Bar F et al. Rivastigmine treatment for the prevention of electroconvulsive therapy-induced memory deficits in patients with schizophrenia. Clin Neuropharmacol. 2012 Jul-Aug;35(4):161-4.
[4]. Han HJ, Lee JJ, Park SA et al. Efficacy and safety of switching from oral cholinesterase inhibitors to the rivastigmine transdermal patch in patients with probable Alzheimer's disease. J Clin Neurol. 2011 Sep;7(3):137-42.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















