3-bromo-5-phenyl Salicylic Acid (Synonyms: NSC 109116) |
| Catalog No.GC17302 |
3-브로모-5-페닐 살리실산은 인간 20α-하이드록시스테로이드 탈수소효소(AKR1C1)의 강력하고 선택적 억제제이며 AKR1C1에 대해 4nM의 Ki 값을 갖습니다.
Products are for research use only. Not for human use. We do not sell to patients.
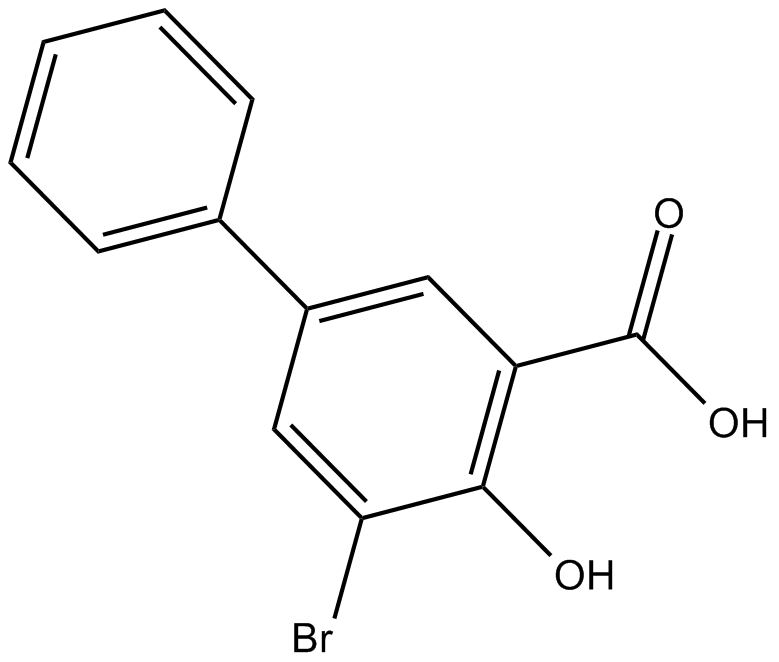
Cas No.: 4906-68-7
Sample solution is provided at 25 µL, 10mM.
Ki: 140 nM for AKR1C1; 1.97 μM for AKR1C2; 21 μM for AKR1C3
3-bromo-5-phenyl Salicylic acid is an AKR1C1 inhibitor.
The aldo-keto reductase (AKR) enzymes are a family of related NADP-dependent oxidoreductases, in which The 1C subfamily (AKR1C) has 4 human hydroxysteroid dehydrogenases (HSD), a 20α-HSD and the other three being 3α-HSDs. AKR1C1 has been found to metabolize progesterone to 20α-hydroxy progesterone, its inactive progestin.
In vitro: In previous screening study, the additional phenyl group of 3-bromo-5-phenylsalicylic acid, targeting a nonconserved hydrophobic pocket in the active site of AKR1C1, resulted in 21-fold improved potency over the structurally similar 3alpha-hydroxysteroid dehydrogenase isoform (AKR1C2). 3-bromo-5-phenyl Salicylic acid was found to be hydrogen bonded to His117, Tyr55, and His222, and the phenyl ring could form additional van der Waals interactions with residues Phe311, Leu308, and Leu54. In addition, the metabolism of progesterone in AKR1C1-overexpressed cells could be potently inhibited by 3-bromo-5-phenylsalicylic acid, which was effective from 10 nM to 460 nM [1].
In vivo: Up to now, there is no animal in vivo data reported.
Clinical trial: So far, no clinical study has been conducted.
Reference:
[1] El-Kabbani, O. ,Scammells, P.J.,Gosling, J., et al. Structure-guided design, synthesis, and evaluation of salicylic acid-based inhibitors targeting a selectivity pocket in the active site of human 20α-hydroxysteroid dehydrogenase (AKR1C1). Journal of Medicinal Chemistry 52, 3259-3264 (2009).
Average Rating: 5 (Based on Reviews and 35 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















