Dorzolamide |
| Catalog No.GC13932 |
Dorzolamide(L671152)는 강력한 탄산 탈수효소 II 억제제로, 적혈구 CA-II 및 CA-I에 대해 IC50 값이 각각 0.18nM 및 600nM입니다. Dorzolamide는 항종양 활성을 가지고 있습니다.
Products are for research use only. Not for human use. We do not sell to patients.
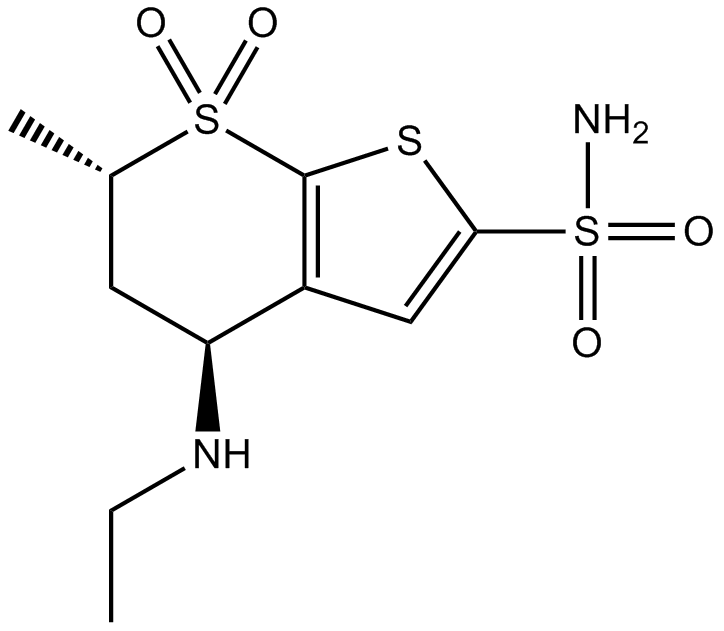
Cas No.: 120279-96-1
Sample solution is provided at 25 µL, 10mM.
Dorzolamide(L671152; MK507) is an anti-glaucoma agent, which is a carbonic anhydrase inhibitor.Target: carbonic anhydrase (CA)Dorzolamide is a carbonic anhydrase inhibitor. It is an anti-glaucoma agent, and acts by decreasing the production of aqueous humour [1]. Glaucoma was induced in the right eye of adult Wistar rats by episcleral venous occlusion. One experimental group was administered dorzolamide 2%-timolol 0.5% combination eye drops, while the other experimental group was administered dorzolamide 2% eye drops. Control groups had surgery without drug administration. Drug application was initiated either 2 weeks before surgery (Group A), from the day of surgery (Group B), 2 weeks after surgery (Group C), or 4 weeks after surgery (Group D). RGCs were labeled by intratectal Fluorogold injections and counted from flat-mount preparations, and IOP was measured using Tonopen. Both dorzolamide-timolol combination and dorzolamide, when applied topically, significantly reduced IOP and improved RGC densities in experimental eyes when compared to control eyes. Earlier initiation, as well as longer duration of drug application, resulted in higher RGC densities [2].Clinical indications: Glaucoma; Ocular hypertensionFDA Approved Date: 1995Toxicity: Dizziness, headache, shortness of breath, slow heartbeat, severe asthma, cardiac arrest
References:
[1]. Rankin AJ, et al. Effects of ocular administration of ophthalmic 2% dorzolamide hydrochloride solution on aqueous humor flow rate and intraocular pressure in clinically normal cats. Am J Vet Res. 2012 Jul;73(7):1074-8.
[2]. Sarup V, et al. Dorzolamide and timolol saves retinal ganglion cells in glaucomatous adult rats. J Ocul Pharmacol Ther. 2005 Dec;21(6):454-62.
Average Rating: 5 (Based on Reviews and 31 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















