Fexinidazole (HOE 239) (Synonyms: HOE239) |
| Catalog No.GC32094 |
펙시니다졸(HOE 239)(HOE 239)은 경구 활성, 강력한 니트로이미다졸 항트리파노솜 약물입니다.
Products are for research use only. Not for human use. We do not sell to patients.
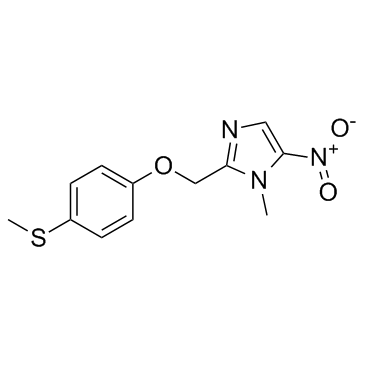
Cas No.: 59729-37-2
Sample solution is provided at 25 µL, 10mM.
Fexinidazole is a 5-nitroimidazole drug currently in clinical development for the treatment of human sleeping sickness (human African trypanosomiasis [HAT]), caused by infection with species of the protozoan parasite Trypanosoma brucei. Target: Antiparasiticin vivo: Fexinidazole shows dose-related efficacy in the T. b. rhodesiense (STIB900) acute mouse model at intraperitoneal (i.p.) doses of 20 to 50 mg/kg/day and oral (per os [p.o.]) doses of 25 to 100 mg/kg/day given on four consecutive days, with 100 mg/kg/day p.o. being 100% curative. Fexinidazole is shown to be effective in the GVR35 mouse model, which mimics the advanced and fatal stage of the disease, when parasites have disseminated into the brain. [1]
[1]. Kaiser M, et al. Antitrypanosomal activity of fexinidazole, a new oral nitroimidazole drug candidate for treatment of sleeping sickness. Antimicrob Agents Chemother. 2011 Dec;55(12):5602-8.
Average Rating: 5 (Based on Reviews and 21 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















