Germacrene D (Synonyms: (-)-Germacrene D) |
| Catalog No.GC40261 |
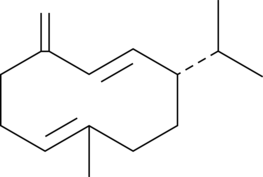
Germacrene D는 Bursera 종에서 분리됩니다. Germacrene D는 항균 및 항진균 활성을 가지며 아미노글리코사이드 및 아졸의 적용에서 보조제로 사용될 수 있습니다.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 23986-74-5
Sample solution is provided at 25 µL, 10mM.
Germacrene D is a major volatile component of Bursera species and a precursor in sesquiterpene biosynthesis in a variety of plants, including Solidago species.[1],[2]
Reference:
[1]. Noge, K., and Becerra, J.X. Germacrene D, a common sesquiterpene in the genus Bursera (Burseraceae). Molecules 14(12), 5289-5297 (2009).
[2]. Bülow, N., and König, W.A. The role of germacrene D as a precursor in sesquiterpene biosynthesis: Investigations of acid catalyzed, photochemically and thermally induced rearrangements. Phytochemistry 55(2), 141-168 (2000).
Average Rating: 5 (Based on Reviews and 40 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















