Ivermectin B1a (Synonyms: dihydro Avermectin B1a,22,23-dihydro Avermectin B1a) |
| Catalog No.GC12700 |
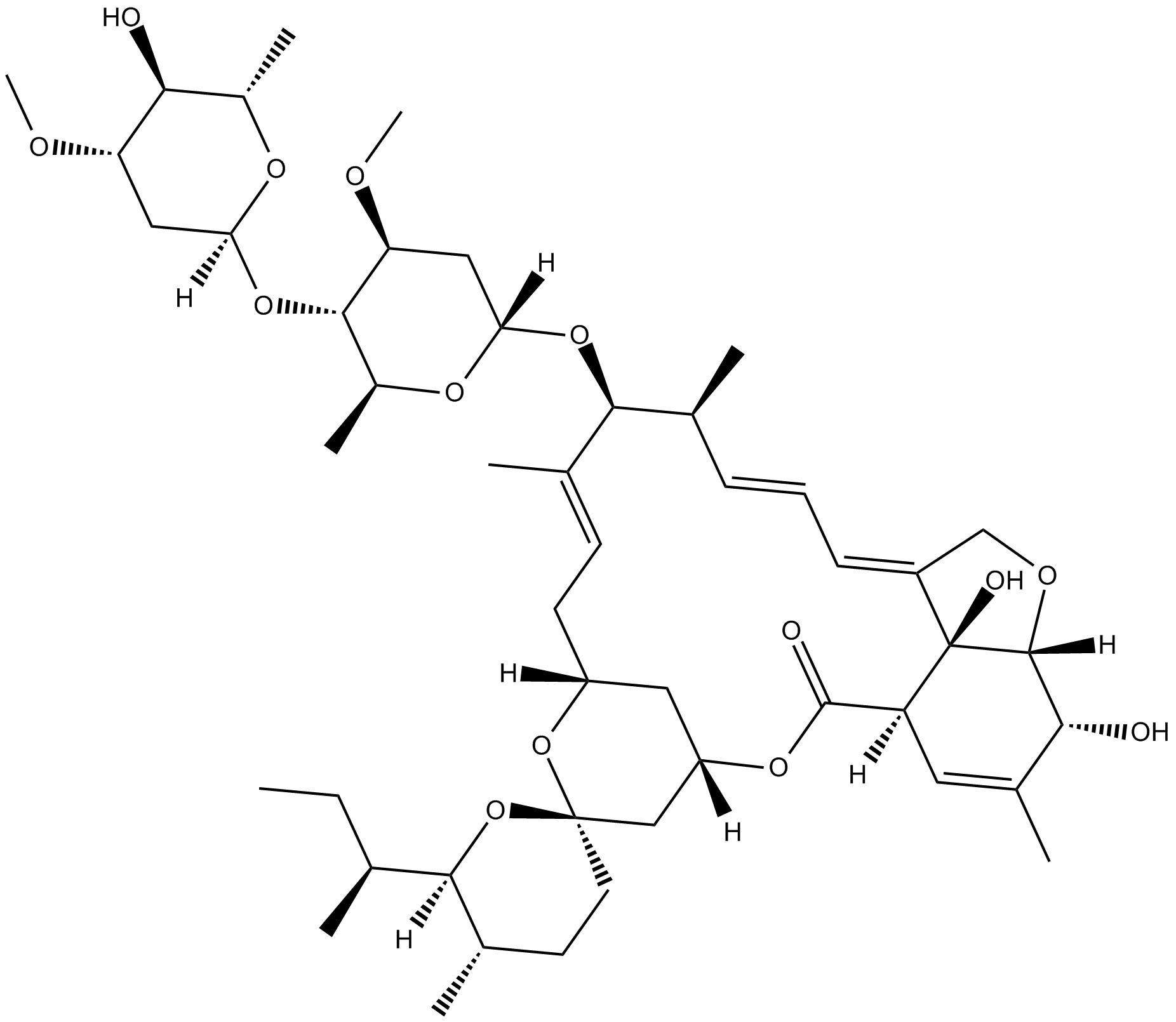
Avermectin B1a의 유도체인 Ivermectin B1a는 Ivermectin의 주성분입니다.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 71827-03-7
Sample solution is provided at 25 µL, 10mM.
Ivermectin B1a is the main component (not less than 80%) of the anthelmintic, ivermectin. Ivermectin is one of the most useful veterinary antiparasitic drugs ever produced. Ivermectin belongs to the macrocyclic lactone class of avermectins. Ivermectin contains two homologous compounds, H2B1a and H2B1b. Avermectins are potent insecticidal, anthelmintic and acaricidal compounds in mediating the paralysis of nematodes and certain classes of ectoparasites by increasing the membrane permeability to chlorine ions[1].
In humans, ivermectin has been used to treat African river blindness (onchocerciasis). Ivermectin significantly decreased the prevalence of skin and eye diseases linked to this infection [2]. Ivermectin B1a produced antiparasitic activity via an interaction with a common receptor molecule, glutamate-gated chloride channels, which virtually expressed on nematode neurons and pharyngeal muscle cells, inducing irreversible channel opening and very long-lasting hyperpolarization/depolarization of the neuron/muscle cell, thereby blocking further function. The EC50 of ivermectin was 104 nM [1,3].
References:
[1] J. Wolsetnholme and A. T. Rogers. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology131 Suppl, S85-S95 (2005).
[2] S. Gaisser, L. Kellenberger, A. L. Kaja, et al. Direct production of ivermectin-like drugs after domain exchange in the avermectin polyketide synthase of Streptomyces avermitilis ATCC31272. Organic & Biomolecular Chemistry 1(16), 2840-2847 (2003).
[3] J. P. Arena, K. K. Liu, P. S. Paress, et al. The mechanism of action of avermectins in Caenorhabditis elegans: Correlation between activation of glutamate-sensitive chloride current, membrane binding, and biological activity. Journal of Parasitology 81, 286-294 (1995).
Average Rating: 5 (Based on Reviews and 37 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















