RD162 |
| Catalog No.GC11957 |
디아릴티오히단토인인 RD162는 경구 활성 비스테로이드성 항안드로겐(NSAA)입니다. RD162는 안드로겐 수용체(AR)에 특이적으로 결합합니다. RD162는 거세 저항성 인간 전립선암의 마우스 모델에서 종양 퇴행을 유도합니다.
Products are for research use only. Not for human use. We do not sell to patients.
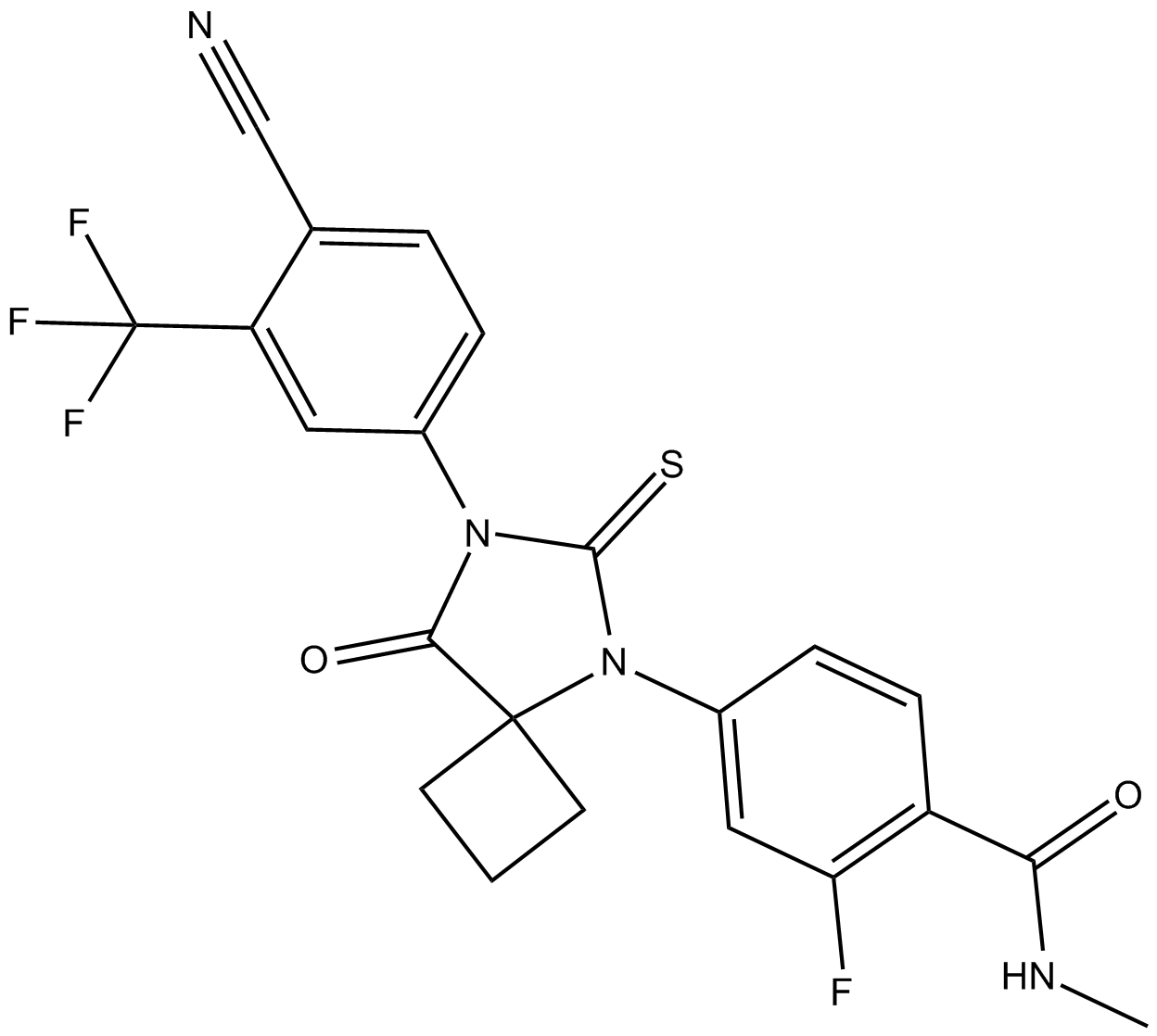
Cas No.: 915087-27-3
Sample solution is provided at 25 µL, 10mM.
IC50: 30.9 nM
RD162 is an androgen receptor (AR) antagonist.
Metastatic prostate cancer is treated with drugs antagonizinf androgen action, but most patients progress to a more aggressive form of the disease named castration-resistant prostate cancer, driven by elevated expression of the androgen receptor (AR).
In vitro: RD162 was optimized from a screen for nonsteroidal antiandrogens retaining activity in the setting of increased androgen receptor expression. RD162 could bind to the androgen receptor with greater relative affinity than the clinically used bicalutamide, reduce the efficiency of the nuclear translocation, and impair both DNA binding to androgen response elements and recruitment of coactivators [1].
In vivo: Previous evidence suggested that the activity of RD162 in these mice was mediated through AR inhibition. The antitumor activity in the LNCaP/AR model was dose-dependent, with some slowing of tumor growth at 0.1 mg/kg RD162 and a few tumor regressions at 1 mg/kg, correlating closely with the effect of these same doses on AR transcriptional activity in the luciferase imaging experiment. In addition, neither bicalutamide nor RD162 impaired the growth of AR-negative DU145 prostate cancer xenografts [1].
Clinical trial: In a phase I/II clinical trial, of the first 30 patients treated with MDV3100, 13 (43%) showed sustained declines in serum concentrations of prostate-specific antigen, a biomarker of prostate cancer [1].
Reference:
[1] C. Tran, S. Ouk, N. J. Clegg, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324(5928), 787-790(2009).
Average Rating: 5 (Based on Reviews and 13 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















