Pharmaceutical Impurities
Products for Pharmaceutical Impurities
- Cat.No. 상품명 정보
-
GC49690
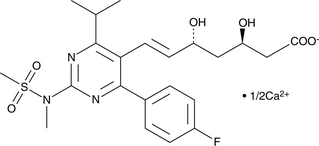
(3R,5R)-Rosuvastatin (calcium salt)
A potential impurity found in bulk preparations of rosuvastatin
-
GC49294
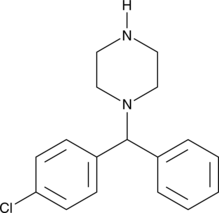
1-(4-Chlorobenzhydryl)piperazine
An inactive metabolite of meclizine and chlorcyclizine
-
GC41935
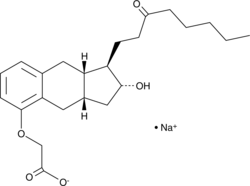
15-keto Treprostinil (sodium salt)
15-keto Treprostinil is an impurity found in treprostinil, which is a stable analog of prostaglandin I2 with a longer plasma half-life.
-
GC42764
Albuterol methyl ether
Salbutamol (albuterol) is a selective β2-adrenergic partial agonist that is used as a bronchodilator.
-
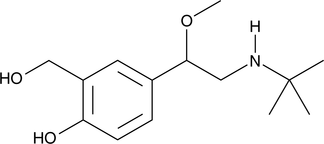
GC49242
Descarbonyl Lacosamide
A potential impurity found in commercial preparations of lacosamide
-
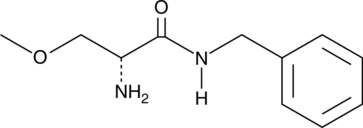
GC49600
Isopropyl 5-(Diphenylphosphoryl)pentanoate
A potential impurity in latanoprost preparations
-
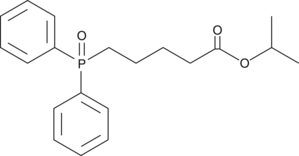
GC43917
Isopropyl Benzenesulfonate
Isopropyl benzenesulfonate is a sulfonate ester genotoxic impurity found in active pharmaceutical ingredients.
-
GC49640
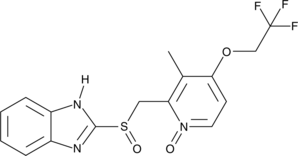
Lansoprazole N-oxide
A potential impurity found in bulk preparations of lansoprazole
-
GC49683
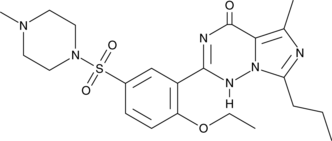
N-desethyl-N-methyl Vardenafil
A potential impurity found in commercial preparations of vardenafil
-
GC49173
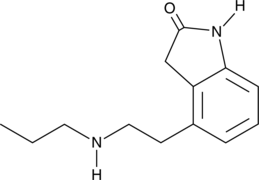
N-Despropyl Ropinirole
An active metabolite of ropinirole
-
GC49249
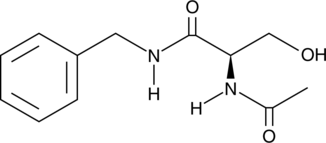
O-Desmethyl Lacosamide
A potential impurity found in commercial preparations of lacosamide
-
GC49095
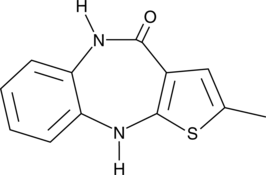
Olanzapine-related Compound B
A potential impurity found in commercial preparations of olanzapine
-
GC49184
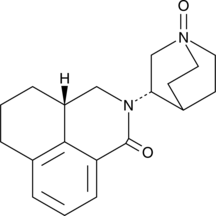
Palonosetron N-oxide
A metabolite of palonosetron
-
GC49663
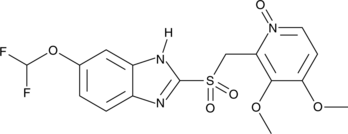
Pantoprazole sulfone N-oxide
A potential impurity found in bulk preparations of pantoprazole
-
GC49171
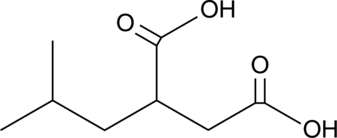
Pregabalin Diacid Impurity
A potential impurity found in commercial preparations of pregabalin
-
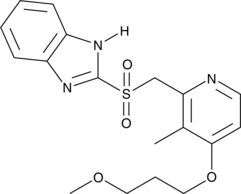
GC49627
Rabeprazole sulfone
A metabolite of rabeprazole
-
GC49653
Ramipril Diketopiperazine
A potential impurity found in commercial preparations of ramipril

-
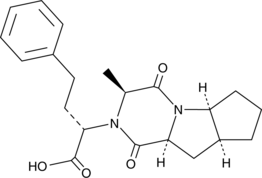
GC49408
Ramipril Diketopiperazine Acid
A potential impurity found in commercial preparations of ramipril
-
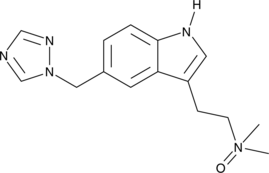
GC49413
Rizatriptan N-oxide
A potential impurity found in commercial preparations of rizatriptan
-
GC49358
Sildenafil Chlorosulfonyl
An intermediate in the synthesis of sildenafil

-
GC49247
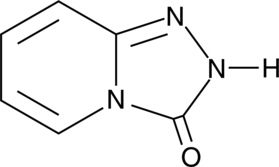
Triazolopyridinone
An intermediate in the synthesis of trazadone