Ixabepilone (Synonyms: Azaepothilone B, BMS-247550) |
| Catalog No.GC16869 |
A broad-spectrum anticancer agent
Products are for research use only. Not for human use. We do not sell to patients.
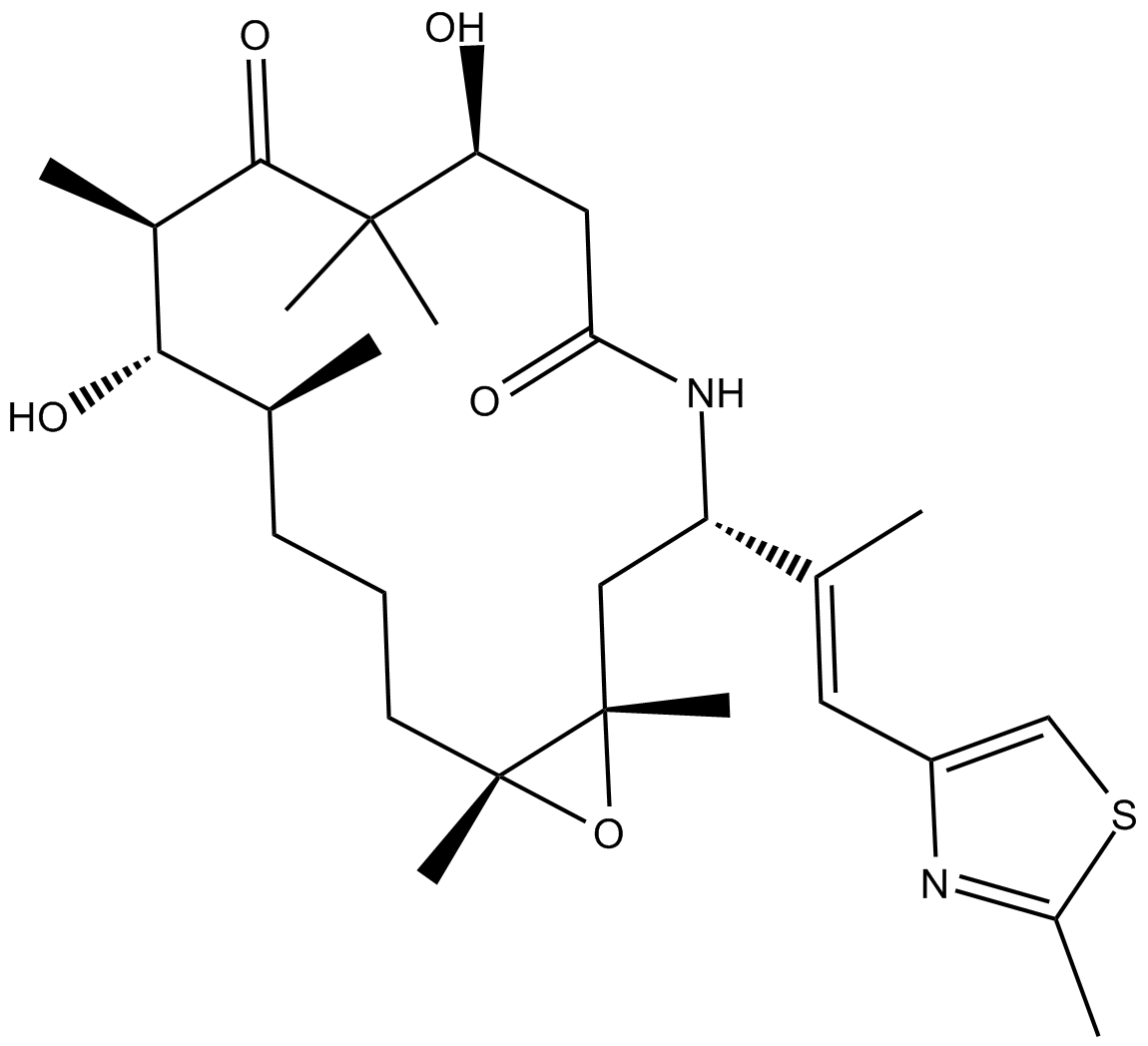
Cas No.: 219989-84-1
Sample solution is provided at 25 µL, 10mM.
Ixabepilone(BMS-247550), an epothilone B analog, is an orally bioavailable microtubule inhibitor [1]. It could bind to tubulin and promote tubulin polymerization and microtubule stabilization, thereby arresting cells in the G2-M phase of the cell cycle and inducing tumor cell apoptosis[2].
In vitro: In a large panel of tumor cell lines, BMS-247550 was as active as epothilone B in inducing cytotoxicity. Of the 21 cells lines tested, the IC50 values were in the range of 1.4–34.5 nm. BMS-247550 almost completely blocked cells in mitosis as early as 8 h after the initiation of drug exposureat a concentration close to the IC90 (7.5 nm, clonogenic cytotoxicity assay) [2].
In vivo: BMS-247550 has clearly demonstrated antitumor activity that is superior to paclitaxel in both paclitaxel-sensitive and -resistant tumors. BMS-247550 was more efficacious than paclitaxel in all five paclitaxel-resistant tumors such as the clinically derived paclitaxel resistant Pat-7 ovarian carcinoma, the A2780Tax ovarian carcinoma that is resistant to paclitaxel because of tubulin mutations, the HCT116/VM46 MDR colon carcinoma, the clinically derived paclitaxel-resistant Pat-21 breast carcinoma, and the murine fibrosarcoma M5076. BMS-247550 produced antitumor activity equivalent to paclitaxel against three paclitaxel-sensitive human tumor xenografts such as A2780 human ovarian carcinoma, HCT116, and LS174T human colon carcinoma [2].
Clinical trials:Numerous phase I trials have evaluated the optimum dose and toxicity associated with ixabepilone. Various phase II studies were designed for patients with varying levels of previous therapies based on ample evidence of response and safety in early trials such as patients with taxane-refractory breast cancer, taxane-naive patients, both taxane-naive and taxane-refractory patients, taxane refractory patients[3].
References:
[1]. Lee FY1, Borzilleri R,Fairchild CR, Kim SH, Long BH, Reventos-Suarez C, Vite GD, Rose WC, Kramer RA.BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res.2001 May;7(5):1429-37
[2]. Denduluri N, Low J A, Lee J J, et al. Phase II trial of ixabepilone, an epothilone B analog, in patients with metastatic breast cancer previously untreated with taxanes[J]. Journal of clinical oncology, 2007, 25(23): 3421-3427.
[3]. Shannon Puhalla, Adam Brufsky. Ixabepilone: a new chemotherapeutic option for refractory metastatic breast cancer. Biologics. 2008 Sep; 2(3): 505–515.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















