Poly(I:C)
Polyinosinic-polycytidylic acid, or poly(I:C), is a synthetic substitute for double-stranded RNA (dsRNA). Poly(I:C) is made up of lengthy chains connecting repeating units of cytidine (C) and inosine (I). It is produced in a manner that closely resembles the structure of viral dsRNA, which frequently sets off the immune system's antiviral reaction. There are several variations of Poly(I:C) that have different properties and uses, such as low molecular weight (LMW) and high molecular weight (HMW) varieties. The molecular structure of poly(I:C) is shown in Figure 1.
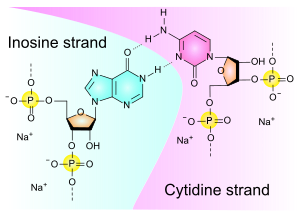
Figure 1: The molecular structure of Poly (I:C).
Polyinosinic-polycytidylic acid (poly(I:C)), has been thoroughly investigated for use in tumor immunotherapy. The human innate immune system is stimulated by polyicin, which then regulates adaptive immunity. This results in changes to the tumor microenvironment and a notable inhibition of tumor growth. For many years, poly(I:C) has been researched in relation to immunotherapy against cancer. Only lately, however, have TLR3, RIG-I, and MDA-5 been identified as the primary receptors implicated in its identification. RLRs are widely expressed in most tissues and are dispersed throughout the cytoplasm, where they recognize intracellular dsRNA, but TLR3 expression is limited to particular cell types, including immune and non-immune cells. TLR3 binds to extracellular and endosomal dsRNA. These two types of receptors have the ability to trigger an immune reaction in response to manufactured dsRNA and nucleic acids produced from viruses .
TLR3 has identified poly(I:C) as the most powerful type I interferon (IFN) inducer. TLR3's subcellular location appears to vary depending on the kind of cell. Although TLR3 expression has been found on fibroblasts' cell surfaces, it is primarily found in intracellular locations like the early endosome or endoplasmic reticulum in macrophages, myeloid dendritic cells (DCs), T cells, natural killer (NK) cells, and other non-immune cells like alveolar epithelial cells, intestinal epithelial cells, hepatoma cells, and melanoma cells. When poly(I:C) binds to TLR3, it starts a signaling pathway in immune cells that recruits TIR-domain-containing adapter-inducing interferonβ (TRIF) and activates transcription factors such as activator protein 1, nuclear factor kappaB (NFκB), and interferon regulatory factor 3 (IRF3). This results in the production of inflammatory cytokines and the induction of type I IFN. Type I IFN has the ability to stimulate DC maturation, increase antigen-specific CD8+ T cell responses, and activate natural killer cells. Conversely, it has been shown that apoptosis is induced by TLR3 or TRIF activation via the Fas-associated protein death domain-caspase-8 signaling pathway. In certain cancer cells, TLR3 activation by poly(I:C) may directly cause apoptosis via extrinsic (caspase-8) and intrinsic (caspase-9, bcl-2 apoptosis-related protein) apoptotic pathways .
It has been demonstrated by recent research that MDA5 and RIG-I are significant IFN-related innate immune receptors. Numerous cells, including immune and non-immune cells such DCs, macrophages, and fibroblast cells, express RIG-I and MDA-5 in their cytoplasm. The downstream molecule that detected polyic and triggered an immune response was RIG-I. Other research, however, has shown that MDA-5—rather than RIG-I—is the essential component in poly(I:C) recognition. A DExD/H box RNA helicase with a caspase recruitment domain is encoded by RIG-I. The transcription factors NFκB and IRF-3 are activated as a result of "downstream" signals transmitted by the caspase recruitment domain through the caspase recruiting domain containing IFN-β promoter stimulator (Cardif). Apoptosis and the suppression of cell proliferation are processes that are mediated by MDA5 and RIG-I . Apoptotic protease activating factor 1 (Apaf-1) and caspase-9 are required for the apoptosis that is initiated by RIG-I and MDA5. Moreover, pro-apoptotic Bcl-2 protein family members Noxa, Puma, Bik, and Bim exhibit elevated expression during RIG-I/MDA-5-mediated apoptosis. Figure 2 illustrates the anticancer mechanisms of poly(I:C).
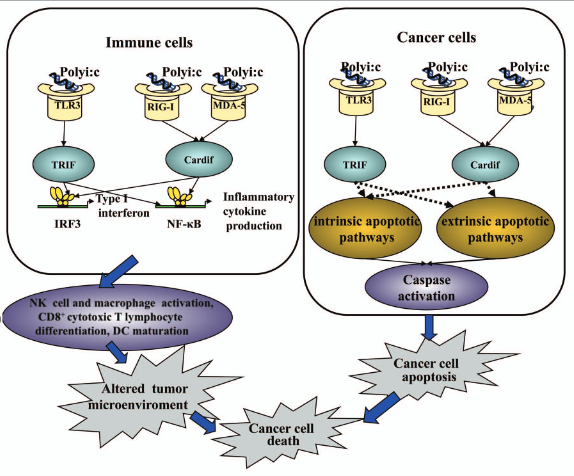
Figure 2: TLR3- and RLR-dependent signaling pathways mediating the anticancer effects of poly(I:C).
Melanoma cells have intracellular localization of TLR3, which is capable of delivering proapoptotic and antiproliferative signals. Polyicin stimulates the TLR3 pathway, which suppresses the viability of melanoma cells as shown in Figure 3. Pretreatment with IFNα may improve this impact in a synergistic manner. TLR3-mediated recognition of poly(I:C) directly initiates apoptosis in cancer cells by activating caspase pathways, including caspase-8/caspase-9, caspase-3, and the mitochondrial apoptotic pathway. In an in vivo investigation, poly(I:C) therapy promoted DC maturation and raised the number of tyrosinase-related protein-2 (TRP-2)-tetramer-positive CD8+ cells. After receiving polyic therapy, intratumoral injection of immature DCs boosted the quantity of TRP-2-specific IFNr-producing cells in the spleen and lymph nodes, thereby enhancing the anticancer immune response. An additional study showed that, in human melanoma cells, poly(I:C) binding to RIG-I/MDA-5 activated both internal (caspase-9, Noxa, Puma, Bik, and Bim) and extrinsic (caspase-8) apoptotic pathways, independent of type I IFN. Furthermore, poly(I:C)'s stimulation of the RIG-I/MDA-5 pathway is necessary for NK cells to operate normally .
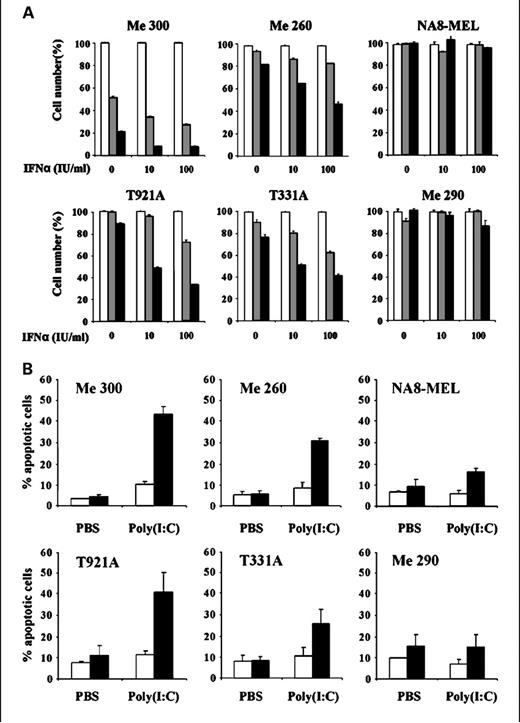
Figure 3: The TLR3 agonist poly(I:C) can alter the viability of melanoma cells.
Human breast cancer cells were subjected to polyicin-induced apoptosis, which was TLR3-dependent and necessitated the generation of IFNβ as shown in Figure 4. TLR3-induced cancer cell apoptosis was mediated by extrinsic caspase pathways, such as caspase-8 and caspase-3, and was independent of dsRNA-dependent protein kinase and myeloid differentiation primary response gene-88.The specific proapoptotic signaling pathways induced by TLR3 in human breast cancer cells need more research, even though poly(I:C) caused cell death in human breast cancers and revealed a pathway downstream of TLR3 through interleukin-1 receptor-associated kinase 4 in the absence of TNF receptor associated factor-6. It is crucial for NK cells to perform their regular tasks in order to prevent cancer cell metastases from spreading and growing . Both in people and animals, surgical stress has been shown to reduce NK cell activation. In a rat model of lung metastasis from mammary adenocarcinoma, repeated low-dose poly(I:C) administration shielded marginating pulmonary NK cells against surgical suppression. A prior study demonstrated that poly(I:C) could stop the spread of breast cancer in a mouse model; however, due to poly(I:C)'s significant toxicity, preclinical and clinical research on the drug's antitumor efficacy has been constrained .
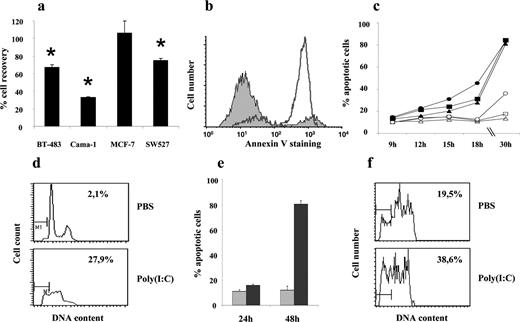
Figure 4: Synthetic dsRNA induces TLR3 dependent apoptosis of human breast tumor cells.
One of the most prevalent diseases is prostate cancer, for which there is presently no cure once metastases and androgen resistance take hold. It has been found that two human prostate cancer cell lines, LNCaP and PC3, express TLR3 functionally. Poly(I:C) produces apoptosis that is dependent on caspase-8 and caspase-3, decreases cell growth, and stimulates mitogen-activated protein kinases (MAPK). Protein kinase C-α signaling pathway activation may be the fundamental mechanism via which poly(I:C) promotes apoptosis in prostate cancer cells. In a mouse model of prostate cancers, activation of TLR3 by repeated poly(I:C) therapy could significantly inhibit the growth of prostate cancer. TLR3-mediated tumor suppression was significantly aided by the activation of NFκB, MAPK, IRFs, and enhanced T and NK cell infiltration inside the tumor microenvironment .
A versatile synthetic molecule with critical applications in molecular biology, immunology, and medicine is poly(I:C). It has shown to be an invaluable tool for research, vaccine development, and the investigation of new therapeutic alternatives due to its capacity to mimic viral RNA and elicit immunological responses. The uses of Poly(I:C) are anticipated to increase as knowledge of RNA biology and immune responses deepens, resulting in novel discoveries in the domains of immunology and medicine.











Commentaires