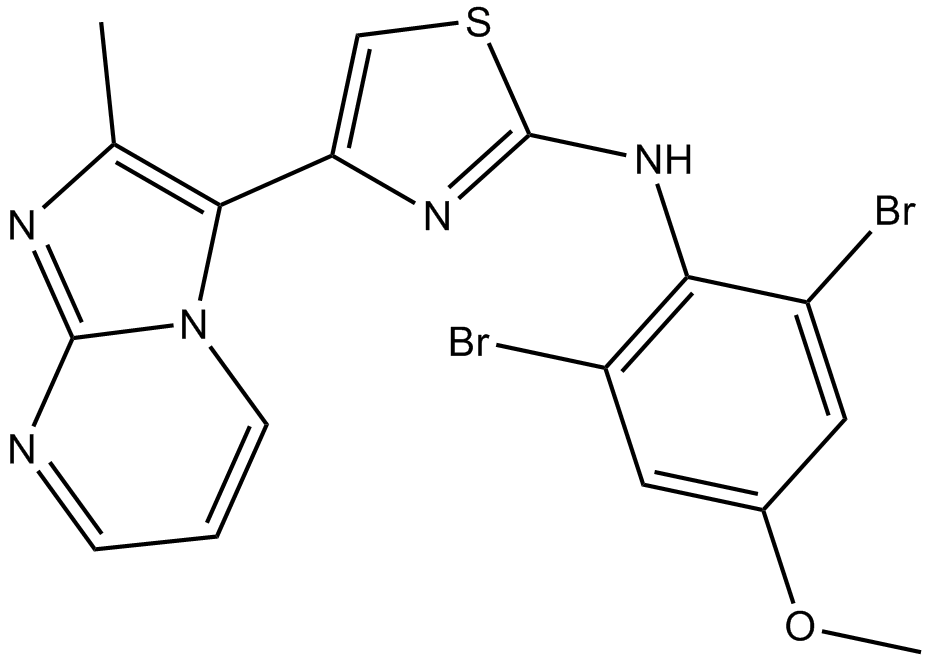
PTC 209
Chemically, PTC 209 is a novel, synthetic and small molecule. Its molecular structure is diagrammatically shown in Figure 1.
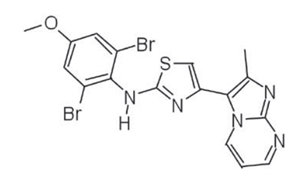
Figure 1 The Molecular Structure of PTC 209
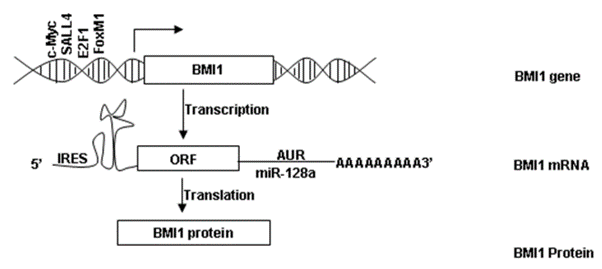
Figure 2 The expression of BMI-1 includes transcription into mRNA followed by translation of the Open Reading Frames ORFs into BMI-1 protein
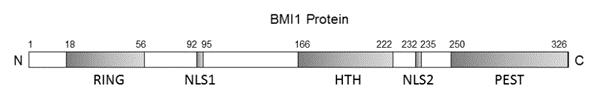
Figure 3 The schematic representation of the BMI-1 protein
It inhibits BMI-1, that is a protein, in a highly specific manner. The BMI-1 protein is expressed by almost every type of cell. The expression of BMI-1 is schematically presented in Figure 2. BMI-1 is the short form of B‐cell‐specific Moloney murine leukemia virus integration site 1 or B lymphoma Mo-MLV insertion region 1 homolog. The functional domains of BMI-1 are shown in Figure 3. On its C terminal, it has Proline (P), Glutamic acid (E)-, Serine (S)- and Threonine (T)-rich domain (PEST), while on its N terminal, it has RING finger domain which is then composed of a three-stranded β-sheet, two zinc binding loops, and an α-helix. The middle domain is Helix-turn-helix (HTH) domain. The Proline (P), Glutamic acid (E)-, Serine (S)- and Threonine (T)-rich domain (PEST) is important for its own regulated turnover. The RING finger domain enables the interaction between BMI-1 and RING1A/B protein and also enables the protein to localize on the broken DNA strands and contribute to DNA damage response. The Helix-turn-helix (HTH) domain enables the interaction between BMI-1 and E4F1. Besides these three domains, the BMI-1 also has two Nuclear localization signals namely, Nuclear localization signal 1 (NLS1) and Nuclear localization signal 2 (NLS2). It has been experimentally shown that removal of Proline (P), Glutamic acid (E)-, Serine (S)- and Threonine (T)-rich domain (PEST) leads to the promotion of the epithelial-to-mesenchymal transition (EMT).
Moreover, it is also called as stem cell gene or stem cell factor; therefore, by effectively targeting it more resistant and relapsing (that has the potential to regrow after medical treatment) type of tumor cells which are also known as cancer stem cells or tumor initiating cells can be effectively treated. Furthermore, this BMI-1 protein is a key member of a protein complex namely Polycomb Repressive Complex 1 (PRC1) which is epigenetic repressor complex. It essentially means that this multi protein complex suppresses the expression of several genes at the transcription stage via bringing changes to the structure of the chromatin as well as bring modifications in the histone proteins. Histone H2A mono-ubiquitination of the residual lysine amino acid at the 119th position, is the common mode of action for this multi protein complex. Methylation of CpG islands is another mode of action of Polycomb Repressive Complex 1 (PRC1); this takes place by getting associated with the promotor region of the methyltransferase 1 (DNMT1). In short, BMI-1 indirectly, being a part of Polycomb Repressive Complex 1 (PRC1) controls the expression of several genes in the cell, more importantly Ink4a locus gene which encodes for p16Ink4a and p14Arf protein. Both of these proteins have the tumor suppressing roles. These mechanisms along with others ones are collaboratively involved in the formation of the tumors; these mechanisms are summarized in Figure 4.
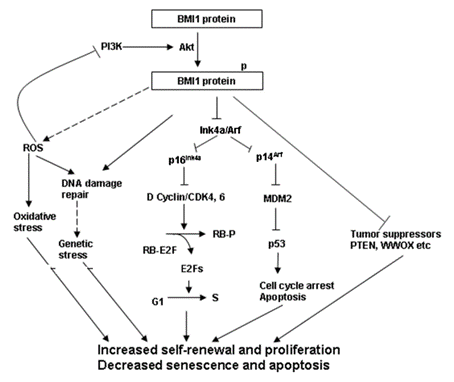
Figure 4 The Signaling Pathways that are found to be involved in the formation of tumors
The composition of the Polycomb Repressive Complex 1 (PRC1) varies from cell to cell and it is still unknown that how this varying composition is responsible for the suppression of the gene transcription in various cells. Yet, it is found to be composed of other proteins as well namely MEL18, RING1A/B, PH1, PH2, and CBX2, CBX4, CBX6, CBX7, CBX8. The way it gets regulated in the cell is still not known. The Polycomb Repressive Complex 1 (PRC1) recognizes the H3K27 trimethylation mark due to the presence of the chromodomains in the different CBX proteins in this complex and thereby this Polycomb Repressive Complex 1 (PRC1) becomes associated to the chromatin. In this way, this Polycomb Repressive Complex 1 (PRC1) is enabled to the interact and modify the chromatin. BMI-1 protein being a part of this huge complex, i.e., Polycomb Repressive Complex 1 (PRC1) significantly enhances the E3 ligase activity of another protein of that complex which is RING1B.
As stated above that the BMI-1 protein brings changes to the gene expression profile of the cells, thus almost all the core cellular processes, that are happening during the development, are affected namely the cell cycle, ageing, apoptosis, angiogenesis, and self-renewal of adult stem cells of different lineages.
The abnormally upregulated expression of BMI-1 protein is a common condition in a number of cancers/ neoplasms therefore, elevated expression of BMI-1 protein is clinically considered as a biomarker of the different type of cancers. Thus, it can be regarded as an oncogene and its status is confirmed in the laboratory in vitro conditions as well as in the laboratory in vivo condition, i.e., animal models. As an oncogene, it leads to the transformation of normal proliferating cells into the abnormally proliferating cancerous cell. In addition, it also promotes the growth of the tumor itself. This experimentally observed fact is graphically presented in Figure 5, taking breast cancer as an example. This high level of BMI-1 is found to be correlated with extremely poor prognosis as it strongly contributes to the formation of tumors, a process named as tumorigenesis. But in some cases, just the overexpressed BMI-1 alone is not sufficient to transform the cell; rather activated H-Ras (RasG12V) along with the overexpression of BMI-1 is necessary to transform the cell. In that case, the underlying mechanism is marked by the dysregulation of numerous growth pathways which are inherently independent of the INK4A/ARF locus. Therefore, for the former case, it is estimated as a potential target protein for the treatment of these broad range of cancerous condition namely ovarian cancer, brain cancer, specifically Glioblastoma multiforme (GBM), head neck squamous cell carcinoma (HNSCC), oral tongue cancer, different breast cancers including human triple negative breast cancer and luminal A‐type breast cancer, prostate cancer, lung cancer, hematologic cancers for example Diffuse Large B cell lymphomas (DLBCL) and acute myeloid leukemia, colorectal carcinoma, non-small cell lung cancer, medulloblastoma, neuroblastomas, gastric gland cancers, biliary tract cancer (BTC).
However, in the experimental setting, it has been shown that reduced levels of BMI-1 protein not only cause the cessation of proliferation in the tumor cells but also cause apoptosis in them which is a type of cell death, a programmed one. In addition, it also enhances the effectiveness of any cytotoxic agent for example, any pharmaceutical drug or radiations
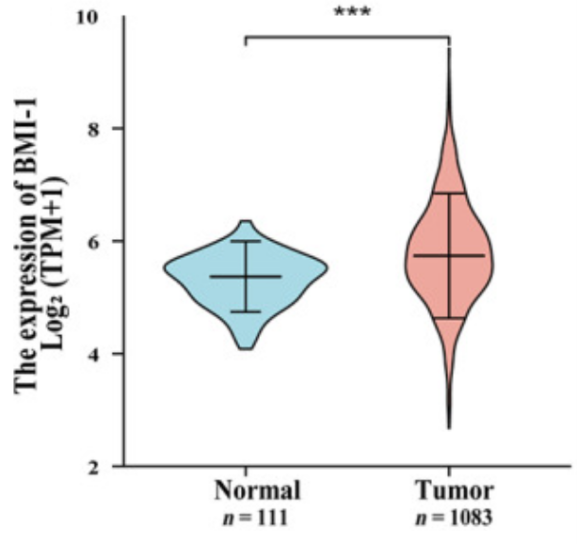
Figure 5 The Abnormally high expression of B lymphoma Mo-MLV insertion region 1 (BMI-1) in the cancerous cells/ tissues of the breast is significantly high and is shown in red graph. On the other hand, the normal/ low expression of B lymphoma Mo-MLV insertion region 1 (BMI-1) in the non-cancerous cells/ tissues of the breast is significantly low and is shown in blue graph.













Commentaires