Apronal (Allylisopropylacetylurea) (Synonyms: Allylisopropylacetylcarbamide, Allylisopropylacetylurea) |
| カタログ番号GC33737 |
筋肉弛緩剤
Products are for research use only. Not for human use. We do not sell to patients.
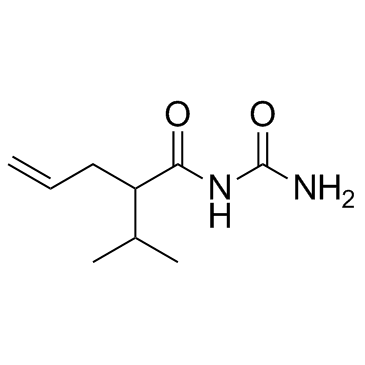
Cas No.: 528-92-7
Sample solution is provided at 25 µL, 10mM.
Apronal (Allylisopropylacetylurea, Apronalide) is a hypnotic and sedative drug that has been withdrawn in several countries due to side effects.
Research shows that allylisopropylacetylurea not only increases the amount of porphyrins in the liver and urine of animals but also brings about a marked green pigmentation of the liver. Rats recover well from long-term treatment with high doses of allylisopropylacetylurea and revert to normal values for parameters measured. Lesions originated by long-term administration with allylisopropylacetylurea can be tolerated by rats and are reversible[1]. Fixed drug eruption and mucocutaneous ocular syndrome are reported due to apronal[2].
[1]. Rentsch G, et al. The effect of prolonged administration of allylisopropylacetylurea to rats on cytochrome P-450 and other liver haemoproteins. Xenobiotica. 1976 Mar;6(3):151-7. [2]. Fujimoto Y, et al. Fixed drug eruption due to allylisopropylacetylurea. Contact Dermatitis. 1993 May;28(5):282-4.
Average Rating: 5 (Based on Reviews and 15 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















