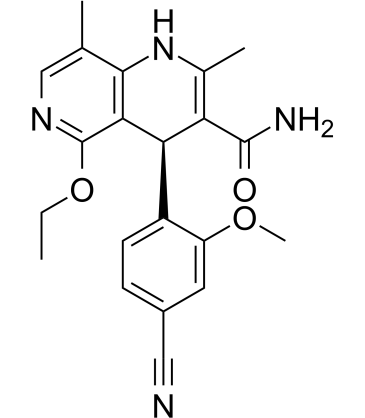
Finerenone (Synonyms: BAY 94-8862) |
| カタログ番号GC60842 |
フィネレノン (BAY 94-8862) は、第 3 世代の選択的経口投与可能な非ステロイド性ミネラルコルチコイド受容体 (MR) アンタゴニスト (IC50=18 nM) です。
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 1050477-31-0
Sample solution is provided at 25 µL, 10mM.
Finerenone (BAY 94-8862) is a third-generation, selective, and orally available nonsteroidal mineralocorticoid receptor (MR) antagonist (IC50=18 nM). Finerenone displays excellent selectivity versus glucocorticoid receptor (GR), androgen receptor (AR), and progesterone receptor (>500-fold). Finerenone has the potential for cardiorenal diseases research, such as type 2 diabetes mellitus and chronic kidney disease[1][2].
Finerenone (BAY 94-8862) lowers albuminuria by >40% and significantly reduces systolic blood pressure (SBP) in Munich Wistar FrÖmter (MWF) rat[1]. Animal Model: Twelve-week-old MWF rat[1]
[1]. BÄrfacker L, et al. Discovery of BAY 94-8862: a nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem. 2012;7(8):1385-1403. [2]. GonzÁlez-BlÁzquez R, et al. Finerenone Attenuates Endothelial Dysfunction and Albuminuria in a Chronic Kidney Disease Model by a Reduction in Oxidative Stress. Front Pharmacol. 2018;9:1131. Published 2018 Oct 9.
Average Rating: 5 (Based on Reviews and 4 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















