(±)19(20)-EpDPA MaxSpec® Standard (Synonyms: (±)19,20 EDP, (±)19,20-epoxy Docosapentaenoic Acid, (±)19,20-epoxy DPA, (±)19,20-EpDPE) |
| カタログ番号GC41200 |
EDHF(内皮由来の過分極因子)は、アセチルコリンとブラジキニンに応答して血管内皮細胞から放出される未確認の仲介物であり、NOS-(一酸化窒素)およびCOX由来の(プロスタサイクリン)拡張剤とは異なります。
Products are for research use only. Not for human use. We do not sell to patients.
Sample solution is provided at 25 µL, 10mM.
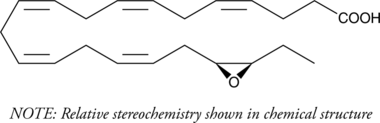
EDHF (endothelium-derived hyperpolarizing factor) is an unidentified mediator released from vascular endothelial cells in response to acetylcholine and bradykinin which is distinct from the NOS- (nitric oxide) and COX-derived (prostacyclin) vasodilators. Cytochrome P450 (CYP450) metabolism of polyunsaturated fatty acids produces epoxides such as (±)14(15)-EpETrE which are prime candidates for the actual active mediator. However, the CYP450 metabolites of eicosapentaenoic acid and docosahexaenoic acid have been little studied relative to arachidonate epoxygenase metabolites. (±)19(20)-EpDPA is a DHA epoxygenase metabolite, derived via epoxidation of the ω-3 double bond of DHA. The EDHF activity of (±)19(20)-EpDPA has not yet been determined. The epoxygenase metabolites of DHA have also been detected in a mouse inflammation model. (±)19(20)-EpDPA MaxSpec® standard is a quantitative grade standard of (±)19(20)-EpDPA that has been prepared specifically for mass spectrometry and related applications where quantitative reproducibility is required. The solution has been prepared gravimetrically and is supplied in a deactivated glass ampule sealed under argon. The concentration was verified by comparison to an independently prepared calibration standard. This (±)19(20)-EpDPA MaxSpec® standard is guaranteed to meet identity, purity, stability, and concentration specifications and is provided with a batch-specific certificate of analysis. Ongoing stability testing is performed to ensure the concentration remains accurate throughout the shelf life of the product. Note: The amount of solution added to the vial is in excess of the listed amount. Therefore, it is necessary to accurately measure volumes for preparation of calibration standards. Follow recommended storage and handling conditions to maintain product quality.
Average Rating: 5 (Based on Reviews and 12 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















