Clindamycin HCl |
| カタログ番号GC15349 |
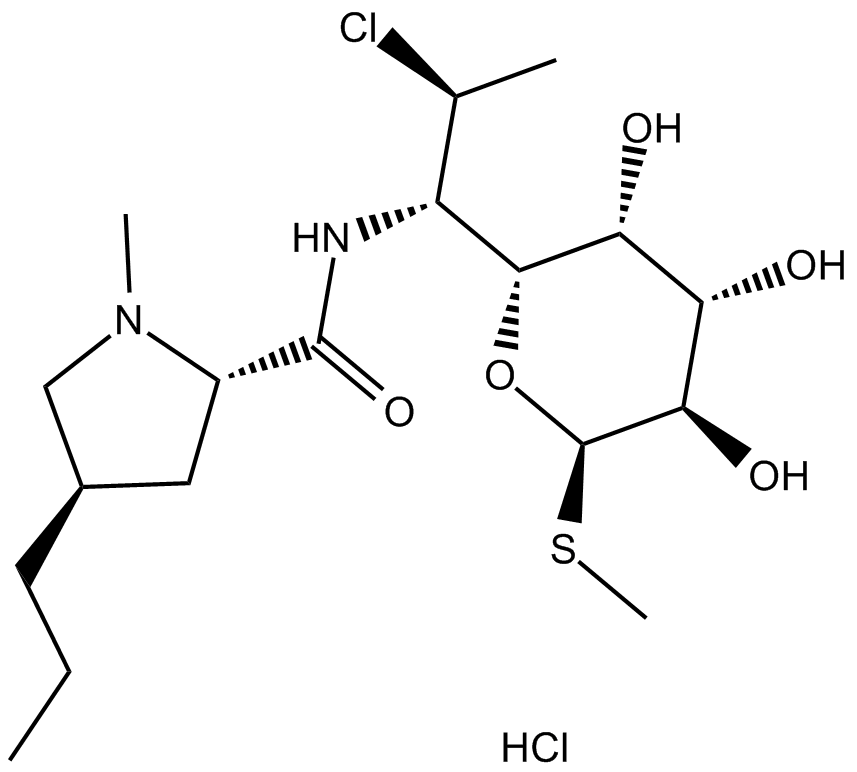
クリンダマイシン (塩酸塩) は、50S リボソームに作用することによってタンパク質合成を阻害する半合成リンコサミド抗生物質です。
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 21462-39-5
Sample solution is provided at 25 µL, 10mM.
Clindamycin (hydrochloride) is a semisynthetic lincosamide antibiotic, which inhibits protein synthesis by acting on the 50S ribosomal.
Clindamycin is a classical inhibitor of bacterial protein synthesis, by binding to the 23S ribosomal RNA of the 50S ribosomal subunit[1].
Clindamycin hydrochloride results in fast absorption after oral administration in dogs, with a mean absorption time (MAT) of 0.87 hour, and bioavailability is 72.55%. Clindamycin hydrochloride results in total clearance (CL) of Clindamycin after both IV and oral administration (0.503 vs. 0.458 L/h/kg) in dogs. Clindamycin hydrochloride results in volume of distribution at steady-state (IV) at 2.48 L/kg, indicating a wide distribution of clindamycin in body fluids and tissues. Clindamycin serum concentrations after IV and oral administration remain above 0.5 μg/mL approximately for 10 hours[1]. Clindamycin hydrochloride significantly reduces oral malodor from the dogs' baseline levels through 42 days. Clindamycin hydrochloride also results in significant reductions in dental plaque, dental calculus, and gingival bleeding in dogs[2]. Clindamycin hydrochloride (2.5 mg/Lb), after ultrasonic scaling, root planing, and polishing (USRP), has a significant effect on plaque and pocket depth measures of periodontal disease but not on gingivitis in canine[3]. Clindamycin hydrochloride results in complete remission ratio of 71.4% (15/21) in dogs with canine superficial bacterial pyoderma after treat within 14 to 28 days[4].
References:
[1]. Batzias GC, et al. Clindamycin bioavailability and pharmacokinetics following oral administration of clindamycin hydrochloride capsules in dogs. Vet J. 2005 Nov;170(3):339-45.
[2]. Warrick JM, et al. Effect of clindamycin hydrochloride on oral malodor, plaque, calculus, and gingivitis in dogs with periodontitis. Vet Ther. 2000 Winter;1(1):5-16.
[3]. Nielsen D, et al. Effects of treatment with clindamycin hydrochloride on progression of canine periodontal disease after ultrasonic scaling. Vet Ther. 2000 Summer;1(3):150-8.
[4]. Bloom PB, et al. Efficacy of once-daily clindamycin hydrochloride in the treatment of superficial bacterial pyoderma in dogs. J Am Anim Hosp Assoc. 2001 Nov-Dec;37(6):537-42.
Average Rating: 5 (Based on Reviews and 37 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















