Eperezolid |
| カタログ番号GC16781 |
エペレゾリド (PNU-100592) はオキサゾリジノン系抗菌剤であり、ブドウ球菌に対するメチシリン感受性 (MIC90= 1-4 mg/ml) に関係なく、エペレゾリドは優れた in vitro 阻害活性を示しました。
Products are for research use only. Not for human use. We do not sell to patients.
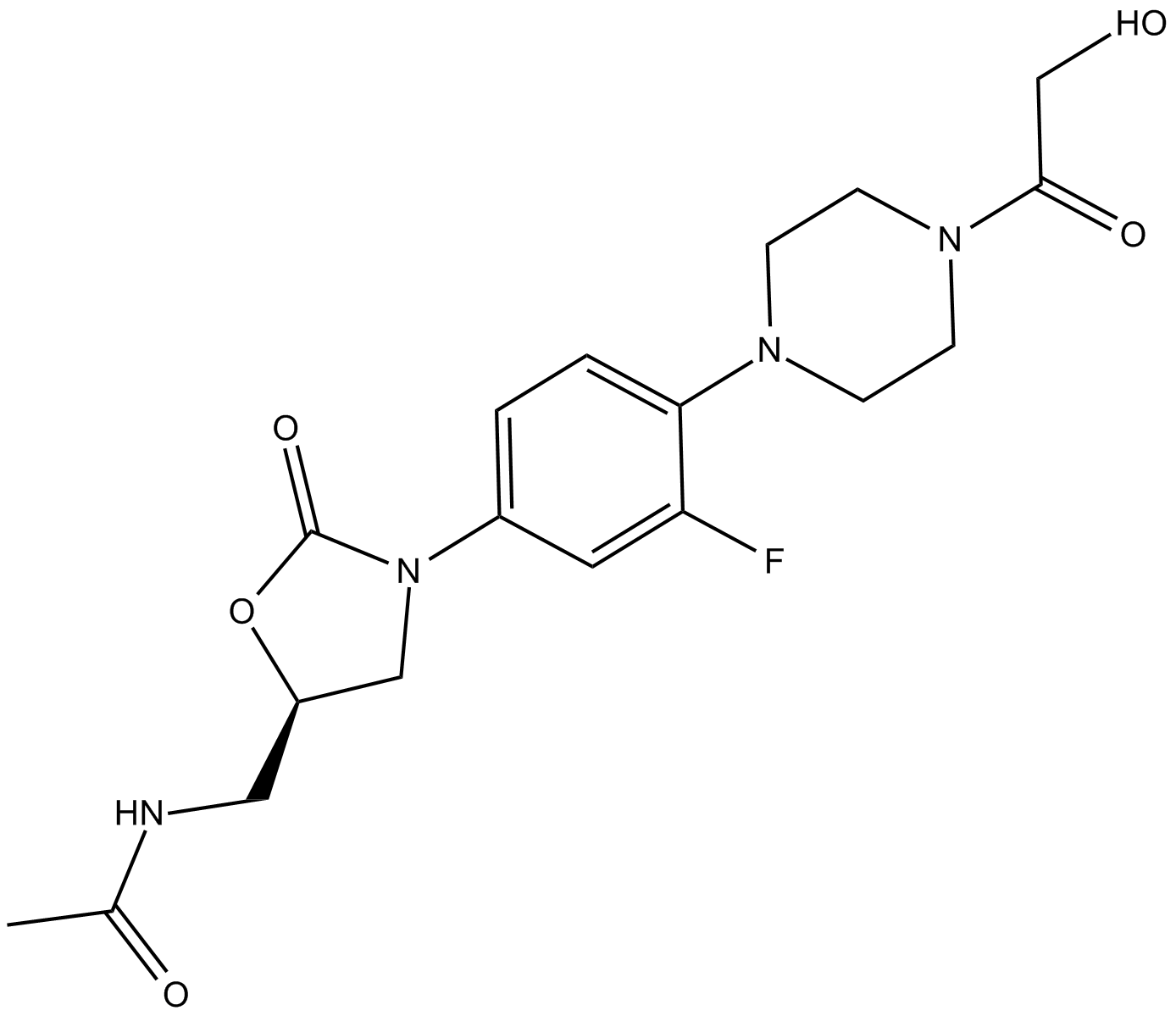
Cas No.: 165800-04-4
Sample solution is provided at 25 µL, 10mM.
MIC50: 0.5, 1.0, 2.0, 1.0, 16.0 and 2.0 mg/L for Pcptostreptococcus, Propionibacterium acnes, Ciostridium pefringens, Clostridium dijiicile, Bactrroidesjagilis, and Fusobacterium, respectivley
Anaerobic bacteria are a common cause of serious infections. Anaerobic species which predominate in clinical infections include the Bacteroides fragilis group, Clostridium spp. and Peptostreptococcus spp. The oxazolidinones are a novel class of synthetic antimicrobials inhibiting the initiation of protein synthesis. Two compounds of this class, eperezolid and linezolid have been shown to inhibit Enterococcus faecalis and Enterococcus faecium.
In vitro: Ninety per cent of all tested Propionibacterium acnes (30 strains), PeptostreptococccuJ spp. (50 strains), C. perjringens (50 strains) and C. dficile (50 strains) were inhibited by
In vivo: The in vivo effectiveness of eperezolid and linezolid against one strain each of Enterococcus faecalis and vancomycin-resistant Enterococcus faecium was examined in a rat intraabdominal abscess model. Eperezolid was ineffective at doses of 25 mg/kg of body weight twice daily for the reductions in abscess bacterial density for E. faecalis. Against E. faecium infections, intravenous eperezolid was effective, reducing densities approximately 2 log10 CFU/g [2].
Clinical trials: Oral administration of eperezolid (1 000 mg PO) to healthy volunteers has earlier been reported to yield peak serum concentration of 6.28 mg/L, respectively, while the trough concentration was estimated to be 1.62 mg/L, respectively [3].
References:
[1] Edlund C, Oh H, Nord CE. In vitro activity of linezolid and eperezolid against anaerobic bacteria. Clin Microbiol Infect. 1999;5(1):51-53.
[2] Schülin T, Thauvin-Eliopoulos C, Moellering RC Jr, Eliopoulos GM. Activities of the oxazolidinones linezolid and eperezolid in experimental intra-abdominal abscess due to Enterococcus faecalis or vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother. 1999;43(12):2873-6.
[3] Schaadt RD, Batts DH, Daley-Yates PT, Pawsey SD, Stalker DJ, Zurenko GE. Serum inhibitory titers and serum bactericidal titers for human subjects receiving multiple doses of the antibacterial oxazolidinones eperezolid and linezolid. Diagn Microbiol Infect Dis. 1997;28(4):201-4.
Average Rating: 5 (Based on Reviews and 25 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















