Otaplimastat (Synonyms: SP-8203) |
| カタログ番号GC63132 |
マトリックスメタロプロテイナーゼ (MMP) 阻害剤であるオタプリマスタット (SP-8203) は、N-メチル-D-アスパラギン酸 (NMDA) 受容体を介した興奮毒性を競合的にブロックします。
Products are for research use only. Not for human use. We do not sell to patients.
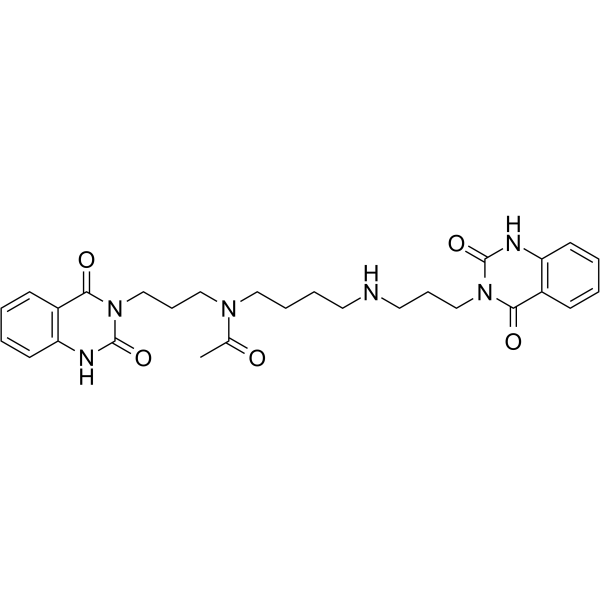
Cas No.: 1176758-04-5
Sample solution is provided at 25 µL, 10mM.
Otaplimastat (SP-8203), a matrix metalloproteinase (MMP) inhibitor, blocks N-methyl-D-aspartate (NMDA) receptor-mediated excitotoxicity in a competitive manner. Otaplimastat also exhibits anti-oxidant activity. Otaplimastat can be used for the research of brain ischemic injury[1][2][3].
Otaplimastat (87.5-350 μM; 20 min) protects neuronal cells against NMDA-induced cell death in a competitive manner[1].Otaplimastat (350 μM) inhibits Ca2+ influx following activation of NMDA receptors in primary cultured neuron[1].Otaplimastat (2-200 μM; pretreated for 4 h) significantly suppresses H2O2-induced cell death and reactive oxygen species production[2].
Otaplimastat (10-20 mg/kg; i.p. 30 min before occlusion and 1 h after reperfusion) prevents ischemic neuronal death in the occlusion model of MCA[1].Otaplimastat (5-10 mg/kg; i.p. daily for 10 days) attenuates impairment of stroke-induced motor function[2].
[1]. Noh SJ, et, al. SP-8203 shows neuroprotective effects and improves cognitive impairment in ischemic brain injury through NMDA receptor. Pharmacol Biochem Behav. 2011 Nov;100(1):73-80.
[2]. Noh SJ, et, al. SP-8203 reduces oxidative stress via SOD activity and behavioral deficit in cerebral ischemia. Pharmacol Biochem Behav. 2011 Mar;98(1):150-4.
[3]. Kim JS, et, al. Safety and Efficacy of Otaplimastat in Patients with Acute Ischemic Stroke Requiring tPA (SAFE-TPA): A Multicenter, Randomized, Double-Blind, Placebo-Controlled Phase 2 Study. Ann Neurol. 2020 Feb;87(2):233-245.
Average Rating: 5 (Based on Reviews and 35 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















