Tolbutamide-d9 (Synonyms: D 860-d9, U-2043-d9) |
| カタログ番号GC48184 |
トルブタミドの定量化のための内部標準
Products are for research use only. Not for human use. We do not sell to patients.
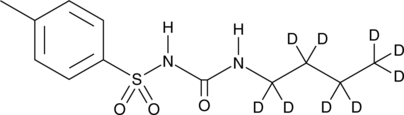
Cas No.: 1219794-57-6
Sample solution is provided at 25 µL, 10mM.
Tolbutamide-d9 is intended for use as an internal standard for the quantification of tolbutamide by GC- or LC-MS. Tolbutamide is an inhibitor of sulfonylurea receptor 1 (SUR1) linked to ATP-sensitive potassium channel Kir6.2 (IC50 = 4.9 µM).1 It is selective for SUR1/Kir6.2 over SUR2A/Kir6.2 and SUR2B/Kir6.2 channels (IC50s = 85 and 88 µM, respectively). Tolbutamide increases glucose-induced insulin secretion and calcium influx in isolated mouse pancreatic islets.2 In vivo, tolbutamide (80 mg/kg) reduces blood glucose levels in a mouse model of diabetes induced by streptozotocin .3 Formulations containing tolbutamide have been used in the treatment of type 2 diabetes.
1.Proks, P., Reimann, F., Green, N., et al.Sulfonylurea stimulation of insulin secretionDiabetes51(3)S368-S376(2002) 2.Ishiyama, N., Ravier, M.A., and Henquin, J.-C.Dual mechanism of the potentiation by glucose of insulin secretion induced by arginine and tolbutamide in mouse isletsAm. J. Physiol. Endocrinol. Metab.290(3)E540-E549(2006) 3.Rerup, C., and Tarding, F.Streptozotocin- and alloxan-diabetes in miceEur. J. Pharmacol.7(1)89-96(1969)
Average Rating: 5 (Based on Reviews and 13 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















