AZD8330 (Synonyms: ARRY-424704; ARRY-704; AZD-8330; ARRY424704; ARRY704; AZD8330) |
| Catalog No.GC14643 |
AZD8330(ARRY-424704)은 IC50이 7nM인 강력하고 경쟁력이 없는 MEK1/MEK2 억제제입니다.
Products are for research use only. Not for human use. We do not sell to patients.
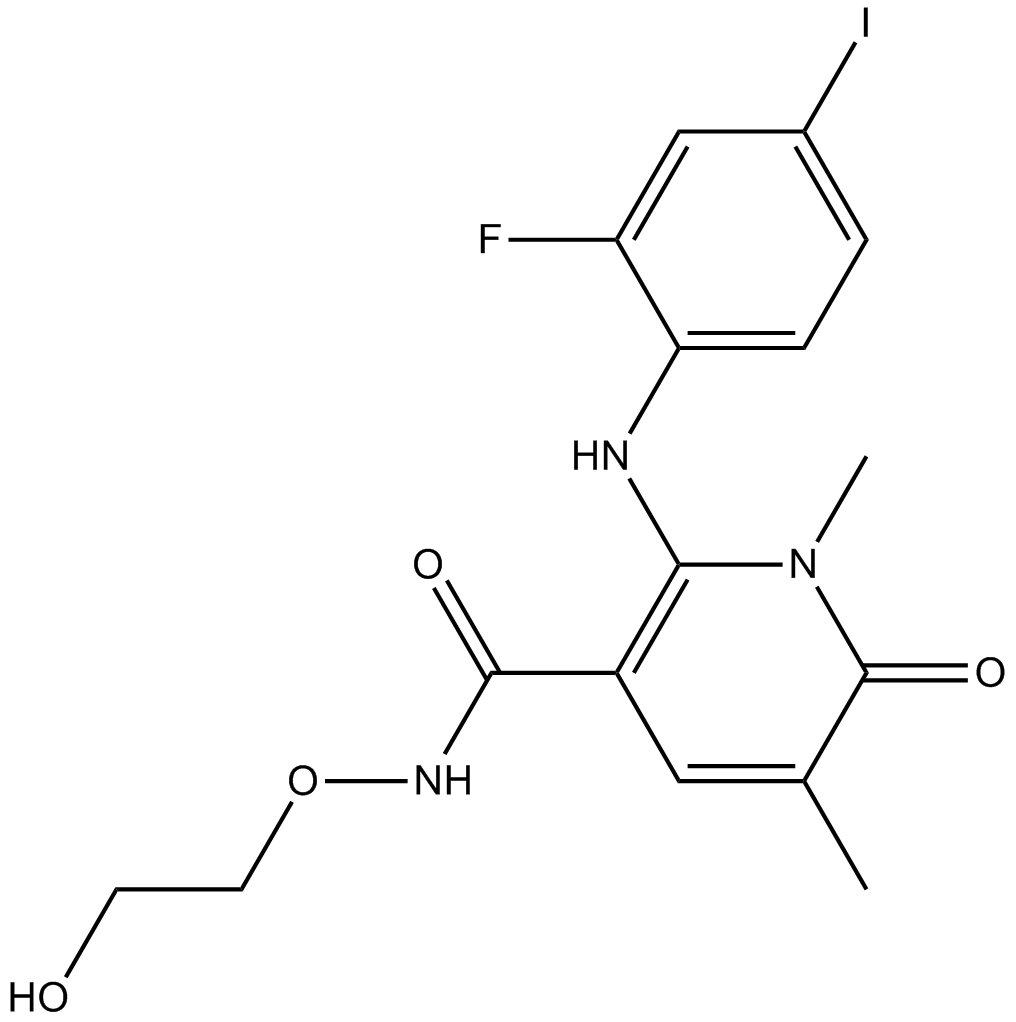
Cas No.: 869357-68-6
Sample solution is provided at 25 µL, 10mM.
AZD8330 is an orally active and selective MEK1/2 inhibitor with an IC50 of 7 nM. The inhibition of MEK1/2 by this component results in inhibition of growth factor-mediated cell signaling and tumor cell proliferation.
MEK, also known as MAP kinase kinase (MAP2K), is a kinase enzyme which phosphorylates mitogen-activated protein kinase (MAPK). The activation of MAPK signaling pathway was shown to be involved in regulation of various cell processes including cell proliferation, differentiation, apoptosis, metabolism and inflammation.
AZD8330 treatment was shown to cause cell apoptosis by suppressing the activation of ERK1/2 signal transduction pathway in Burkitt's lymphoma cell line Raji cells, which is in a dose and time dependent manner [1].
Recently, a large phase I trial of 82 patients with advanced tumor malignancies was conducted to define the maximum tolerated dose (MTD) and assessed the safety, tolerability, pharmacokinetics and pharmacodynamics of AZD8330. The MTD of this product was determined as 40mg/day [2].
References:
1. Feng K, Wang C, Zhou H, Yang J, Dong L, Zhou K, et al. [Effect of ERK1/2 inhibitor AZD8330 on human Burkitt's lymphoma cell line Raji cells and its mechanism]. Zhonghua Xue Ye Xue Za Zhi 2015,36:148-152.
2. Cohen RB, Aamdal S, Nyakas M, Cavallin M, Green D, Learoyd M, et al. A phase I dose-finding, safety and tolerability study of AZD8330 in patients with advanced malignancies. Eur J Cancer 2013,49:1521-1529.
Average Rating: 5 (Based on Reviews and 18 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















