(-)-Cyclopenol (Synonyms: NSC 604990) |
| Catalog No.GC45247 |
(-)-Cyclopenol은 호주 해양에서 분리된 Aspergillus versicolor(MST-MF495)에서 분리된 곰팡이 대사 산물입니다.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 20007-85-6
Sample solution is provided at 25 µL, 10mM.
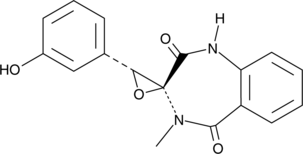
(-)-Cyclopenol is a benzodiazepine alkaloid fungal metabolite originally isolated from P. viridicatum. It inhibits protein tyrosine phosphatase 1B (PTP1B; IC50 = 30 μM).
Review for (-)-Cyclopenol
Average Rating: 5 (Based on Reviews and 36 reference(s) in Google Scholar.)
Review for (-)-Cyclopenol
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















