Mavelertinib (Synonyms: PF-06747775) |
| Catalog No.GC61030 |
마벨러티닙은 선택적이고 경구로 이용 가능한 비가역적 EGFR 티로신 키나제 억제제(EGFR TKI)로, Del, L858R 및 이중 돌연변이 T790M/L858R 및 T790M/Del에 대해 IC50이 각각 5, 4, 12 및 3 nM입니다. Mavelertinib은 비소세포폐암(NSCLC) 연구에 사용할 수 있습니다.
Products are for research use only. Not for human use. We do not sell to patients.
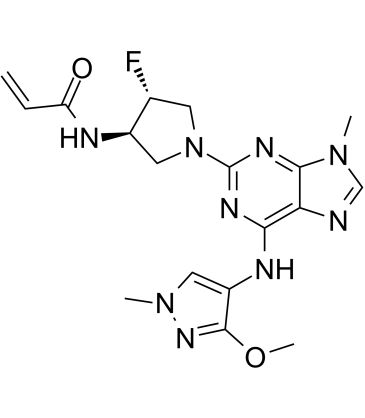
Cas No.: 1776112-90-3
Sample solution is provided at 25 µL, 10mM.
Mavelertinib is a selective, orally available and irreversible EGFR tyrosine kinase inhibitor (EGFR TKI), with IC50s of 5, 4, 12 and 3 nM for Del, L858R, and double mutants T790M/L858R and T790M/Del, respectively. Mavelertinib can be used for the research of non-small-cell lung cancer (NSCLC)[1][2][3][4].
Mavelertinib exhibits selectivity over wild-type EGFR (IC50=307 nM)[1].Mavelertinib (10 μM) exhibits less than 50% e?ect or inhibition against all nonkinase targets[1].Mavelertinib inhibits the hERG26 current with an IC50 > 100 μM[1].
Mavelertinib exhibits low to moderate oral bioavailability (mouse 60%, rat 11%, dog 66%) following oral administration (mouse 1, rat 30, dog 3 mg/kg)[1].Mavelertinib exhibits short plasma half-lives (mouse 0.56, rat 0.28, dog 1.3 h) due to moderate to high plasma clearance (mouse 53, rat 49, dog 12 mL/min/kg) and low steady-state volume of distribution (mouse 1.48, rat 0.66, dog 0.94 L/kg) following intravenous administration (1 mg/kg to mouse, rat and dog)[1]. Animal Model: Female Nu/Nu mice[1]
[1]. Planken S, et, al. Discovery of N-((3R,4R)-4-Fluoro-1-(6-((3-methoxy-1-methyl-1H-pyrazol-4-yl)amino)-9-methyl-9H-purin-2-yl)pyrrolidine-3-yl)acrylamide (PF-06747775) through Structure-Based Drug Design: A High Affinity Irreversible Inhibitor Targeting Oncogenic EGFR Mutants with Selectivity over Wild-Type EGFR. J Med Chem. 2017 Apr 13;60(7):3002-3019. [2]. Murtuza A, et, al. Novel Third-Generation EGFR Tyrosine Kinase Inhibitors and Strategies to Overcome Therapeutic Resistance in Lung Cancer. Cancer Res. 2019 Feb 15; 79(4): 689-698. [3]. Patel H, et, al. Recent updates on third generation EGFR inhibitors and emergence of fourth generation EGFR inhibitors to combat C797S resistance. Eur J Med Chem. 2017 Dec 15; 142:32-47. [4]. Husain H, et, al. First-in-human phase I study of PF-06747775, a third-generation mutant selective EGFR tyrosine kinase inhibitor (TKI) in metastatic EGFR mutant NSCLC after progression on a first-line EGFR TKI. Annals of Oncology. 2017 Sep.
Average Rating: 5 (Based on Reviews and 17 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















