Ondansetron hydrochloride dihydrate |
| Catalog No.GC11651 |
Ondansetron(GR 38032) 염산염 탈수화물은 경구 활성, 고도로 선택적이고 경쟁적인 5-HT3 수용체 길항제입니다(혈액뇌장벽을 통과함).
Products are for research use only. Not for human use. We do not sell to patients.
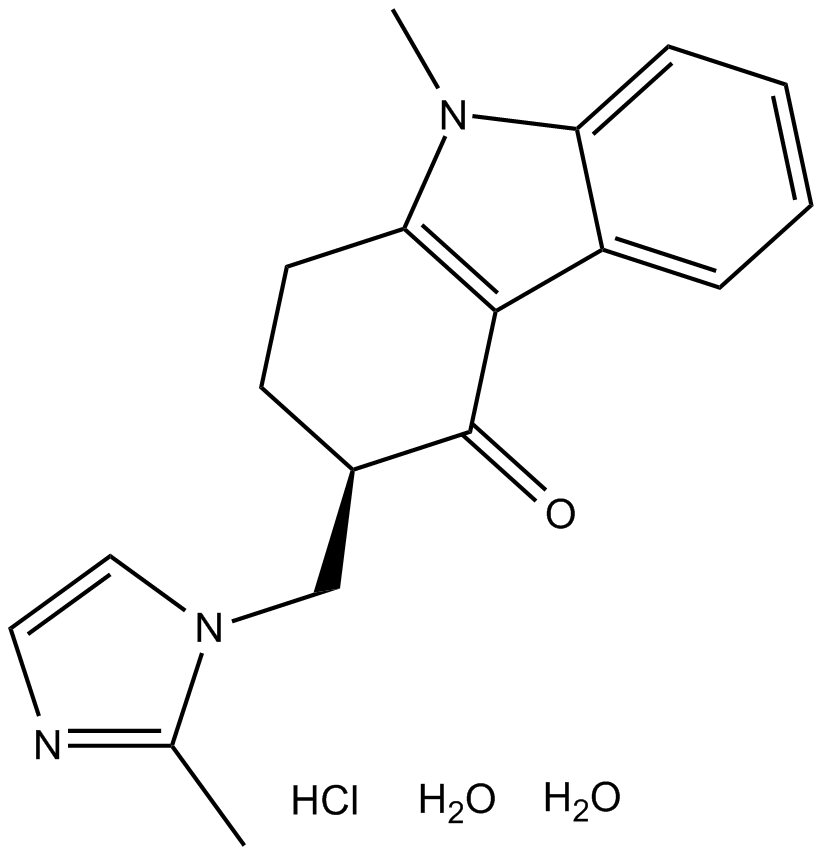
Cas No.: 103639-04-9
Sample solution is provided at 25 µL, 10mM.
Ondansetron hydrochloride dihydrate (GR 38032 hydrochloride dihydrate; SN 307 hydrochloride dihydrate) is a serotonin 5-HT3 receptor antagonist used mainly as anantiemetic (to treat nausea and vomiting), often following chemotherapy.Target: 5- HT3 ReceptorIC50 Value: in vitro: 5-HT evoked transient inward currents (EC50 = 3.4 microM; Hill coefficient = 1.8) that were blocked by the 5-HT3 receptor antagonist ondansetron (IC50 = 103 pM) [1]. The 5-HT3A receptor antagonist ondansetron (0.3 nM) reversibly inhibited the 5-HT (30 microM) signal by 70% and at 3 nM it abolished the response [2].in vivo: Acute ondansetron administration at the lowest dose (0.1 mg/kg, IP) tested had no effect, while other doses (0.33 and 1 mg/kg, IP) produced improvements in auditory gating [3]. Different doses of ondansetron were injected intraperitoneally (i.p.) at fixed times during the day to determine both the sublethal (TD50) and lethal (LD50) doses, which were, respectively, 3.7 +/- 0.6 mg/kg and 4.6 +/- 0.5 mg/kg [4]. ondansetron (0.25-1.0 mg/kg, subcutaneously) given before the challenge dose of ethanol (2.4 g/kg, intraperitoneally) injection, significantly and dose dependently attenuated the expression of sensitization. In addition, ondansetron (1.0 mg/kg, subcutaneously) given before ethanol injection on days 1, 4, 7, and 10 significantly blocked the development (days 1, 4, 7, and 10), and expression (day 15) of sensitization to the locomotor stimulant effect of ethanol injection [5]. Toxicity: Ondansetron may be safe in lower doses used to prevent nausea and vomiting in radiation treatment or postoperatively. However, as there is a report that a lower dose of ondansetron prolonged the QT interval in healthy volunteers, this needs to be clarified by the FDA [6].
References:
[1]. Brown AM, et al. Ion permeation and conduction in a human recombinant 5-HT3 receptor subunit (h5-HT3A). J Physiol. 1998 Mar 15;507 ( Pt 3):653-65.
[2]. Barann M, et al. Recombinant human 5-HT3A receptors in outside-out patches of HEK 293 cells: basic properties and barbiturate effects. Naunyn Schmiedebergs Arch Pharmacol. 2000 Sep;362(3):255-65.
[3]. Wildeboer KM, et al. Ondansetron results in improved auditory gating in DBA/2 mice through a cholinergic mechanism. Brain Res. 2009 Dec 1;1300:41-50.
[4]. Khedhaier A, et al. Circadian rhythms in toxic effects of the serotonin antagonist ondansetron in mice. Chronobiol Int. 2003 Nov;20(6):1103-16.
[5]. Umathe SN, et al. The 5-HT3 receptor antagonist, ondansetron, blocks the development and expression of ethanol-induced locomotor sensitization in mice. Behav Pharmacol. 2009 Feb;20(1):78-83.
[6]. Doggrell SA, et al. Cardiac safety concerns for ondansetron, an antiemetic commonly used for nausea linked to cancer treatment and following anaesthesia. Expert Opin Drug Saf. 2013 May;12(3):421-31.
Average Rating: 5 (Based on Reviews and 6 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















