Quizartinib (AC220) (Synonyms: AC220) |
| Catalog No.GC17615 |
Quizartinib(AC220)(AC220)은 1.6nM의 Kd를 갖는 경구 활성, 고도로 선택적이고 강력한 2세대 FLT3 티로신 키나제 억제제입니다. Quizartinib(AC220)은 각각 4.2 및 1.1 nM의 IC50으로 MV4-11 세포에서 야생형 FLT3 및 FLT3-ITD 자가인산화를 억제합니다. Quizartinib(AC220)은 최적화된 링커를 통해 VHL 리간드에 연결되어 PROTAC FLT3 분해자를 형성할 수 있습니다. Quizartinib(AC220)은 세포 사멸을 유도합니다.
Products are for research use only. Not for human use. We do not sell to patients.
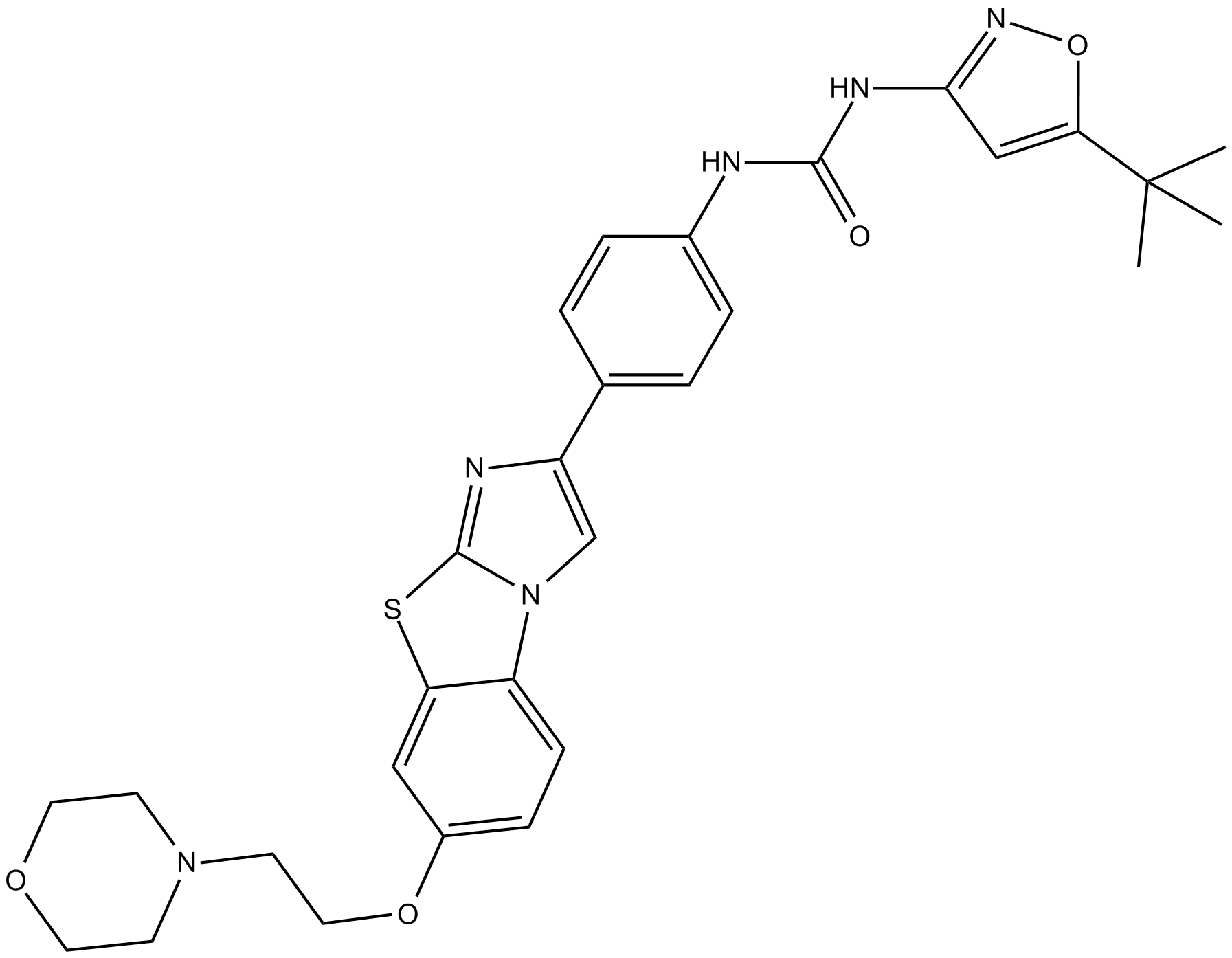
Cas No.: 950769-58-1
Sample solution is provided at 25 µL, 10mM.
Quizartinib (AC220) is a 2nd-generation FLT3 inhibitor for Flt3(ITD/WT) with IC50 value of 1.1 nM/4.2 nM, and it is ten-fold more selective for Flt3 than PDGFRα, PDGFRβ, KIT, RET and CSF-1R [1].
Quizartinib inhibits FLT3 with low nanomolar potency in cellular assays and shows high selectivity when screened against most of the human protein kinome. In addition, the combination of high potency and selectivity exhibited by quizartinib is unique compared with CEP-701, PKC-412, MLN-518, sunitinib, and sorafenib.
Quizartinib (AC220) was identified to be the most potent and selective FLT3 inhibitor with good pharmaceutical properties and superior efficacy in tumor xenograft models. A single dose of 10 mg/kg was administered to mice by oral gavage and plasma levels were measured over a 24-hour period. Quizartinib was well absorbed, achieving a maximum plasma level (Cmax) of 3.8 μM (2100 ng/mL) within 2 hours of dosing. To determine the effect of FLT3-ITD inhibition on cell growth,. These results establish that AC220 has strong activity against FLT3 in biochemical and cellular assays in MV4-11 cell proliferation in the presence of 1.1nM quizartinib [1].
As a FLT3 inhibitor for the treatment of acute myeloid leukemia (AML), when at doses as low as 1 mg/kg orally once a day, quizartinib inhibits FLT3 activity in vivo extending survival significantly. And this eradicates tumors in a FLT3-dependent mouse xenograft model, and potently inhibits FLT3 activity in primary patient cells at a dose of 10 mg/kg. In addition, quizartinib has been demonstrated a desirable safety and PK profile in humans. The emergence of resistant mutations is a common mechanism of resistance to FLT3 inhibitors used clinically, with a mutation emerging in at least 20% of the patients. This shows that the survival of AML blasts depends to a great extent on FLT3 signaling in these cases [2, 3].
References:
[1]. Zarrinkar PP, Gunawardane RN, Cramer MD, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood, 2009, 114(14): 2984-2992.
[2]. Chao Q, Sprankle KG, Grotzfeld RM, et al. Identification of N-(5-tert-Butyl-isoxazol-3-yl)-N'-{4-[7-(2-morpholin-4-yl-ethoxy)imidazo[2,1-b][1,3]benzothiazol-2-yl]phenyl}urea Dihydrochloride (AC220), a Uniquely Potent, Selective, and Efficacious FMS-Like Tyrosine Kinase-3 (FLT3) Inhibitor. Journal of Medicinal Chemistry, 2009, 52(23): 7808-7816.
[3]. Alvarado Y , Kantarjian HM, Luthra R, et al. Treatment With FLT3 Inhibitor in Patients With FLT3-Mutated Acute Myeloid Leukemia Is Associated With Development of Secondary FLT3-Tyrosine Kinase Domain Mutations. Cancer, 2014, 120(14): 2142-2149.
Average Rating: 5 (Based on Reviews and 21 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















