MN-001 (Synonyms: KCA 757, Tipelukast) |
| Catalog No.GC31832 |
MN-001(KCA 757)은 설피도펩티드 류코트리엔 수용체 길항제로서 경구 생체이용 가능한 항염증제로 천식 치료에 사용됩니다.
Products are for research use only. Not for human use. We do not sell to patients.
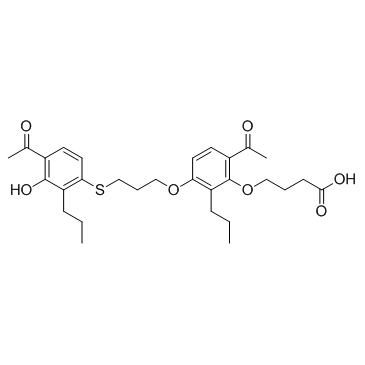
Cas No.: 125961-82-2
Sample solution is provided at 25 µL, 10mM.
Tipelukast (KCA 757) is a sulfidopeptide leukotriene receptor antagonist, an orally bioavailable anti-inflammatory agent and used for the treatment of asthma.
Tipelukast inhibits the binding of [3H] LTD4 to the LTD4 receptors on pul-monary cell membrane of guinea-pigs (IC50 = 2.3 μmol)[2].
Fiftheen min after an aerosolized antigen challenge, and UNDW inhaled 5 min later into the guinea pigs, Tipelukast significantly alters the UNDW-induced bronchoconstriction[1]. Tipelukast (1 and 5 mg/kg) administered intravenously 15 min after antigen challenge reduces the propranolol-induced bronchoconstriction (PIB) in a dose-dependent manner in guinea-pigs[2].
[1]. Fujimura M, et al. No involvement of lipid mediators in a guinea pig model of ultrasonically nebulized distilled water-induced bronchoconstriction. Prostaglandins Other Lipid Mediat. 2000 Jan;60(1-3):49-58. [2]. Fujimura M, et al. Role of leukotrienes in post-allergic propranolol-induced bronchoconstriction in guinea-pigs. Clin Exp Allergy. 1997 Oct;27(10):1219-26.
Average Rating: 5 (Based on Reviews and 26 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















