(Z)-4-Hydroxytamoxifen (Synonyms: ICI 79280, trans-4-hydroxy Tamoxifen) |
| Catalog No.GC16372 |
(Z)-4-히드록시타목시펜은 타목시펜의 대사산물입니다. (Z)-4-히드록시타목시펜은 타목시펜보다 ER에 대해 더 높은 친화도를 나타냅니다. (Z)-4-히드록시타목시펜은 인간 자궁내막 선암종 세포에서 비-세포자멸사 세포독성 효과를 유도합니다.
Products are for research use only. Not for human use. We do not sell to patients.
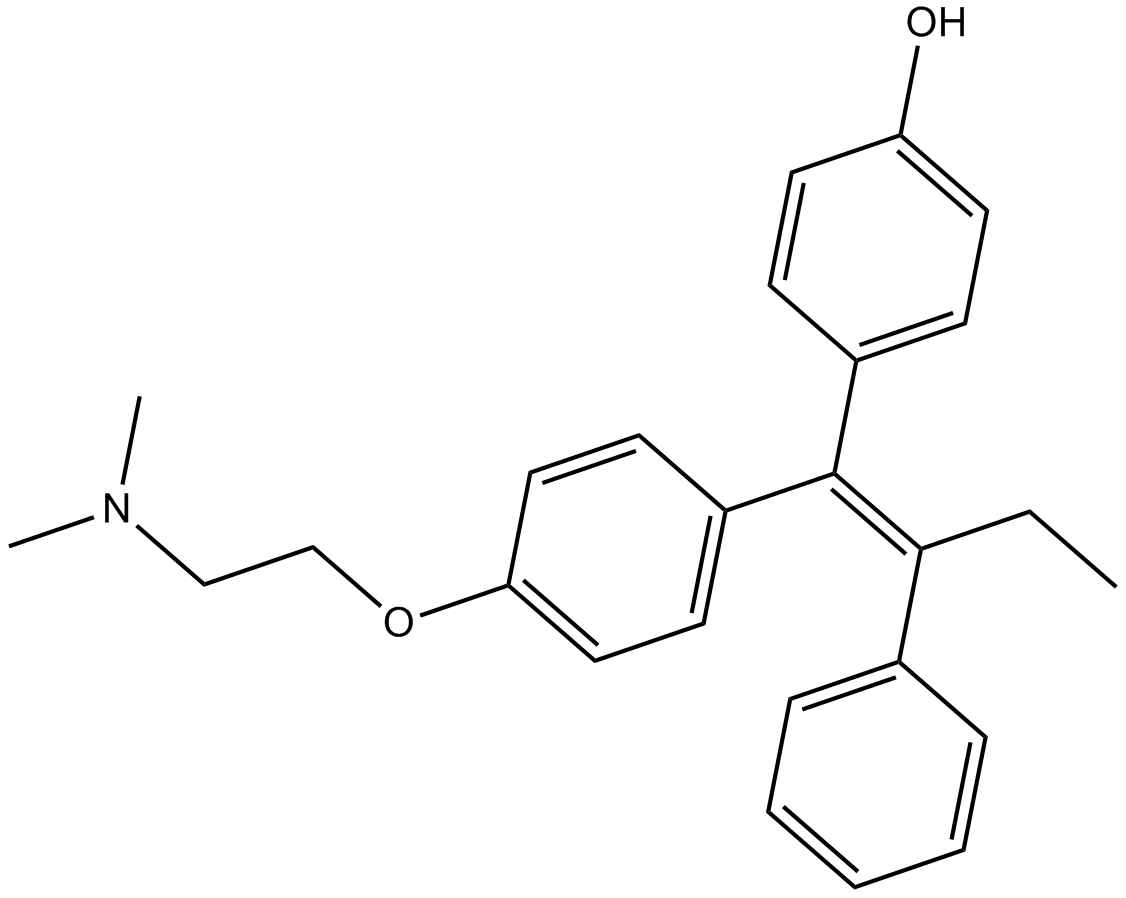
Cas No.: 68047-06-3
Sample solution is provided at 25 µL, 10mM.
(Z)-4-Hydroxytamoxifen is a selective estrogen receptor modulator (SERM) that influences the binding of [3H]oestradiol to the estrogen receptor with an IC50 value of 3.3 nM[1]. (Z)-4-Hydroxytamoxifen can reduce off-target effects in CRISPR-mediated gene editing[2]. It is a metabolite of tamoxifen, targeting estrogen receptors, especially in breast cancer cells, acting as an antagonist in breast tissues and as an agonist in other tissues such as the endometrium. This dual action makes it a key drug in the treatment and chemoprevention of estrogen receptor-positive breast cancer[3].
In vitro, (Z)-4-Hydroxytamoxifen (1-100nM) treatment of immature rat pituitary cells for 6 days inhibits estradiol-stimulated PRL synthesis more effectively than tamoxifen[4]. At low concentrations (0.01-1.00 nM) in the absence of estradiol, (Z)-4-Hydroxytamoxifen significantly stimulates the formation of MCF-7 cell colonies[5].
In vivo, (Z)-4-Hydroxytamoxifen (0.2, 1, 5 µg/d, p.o.) causes a dose-dependent decrease in wet weight of the uterus in immature rats[1]. (Z)-4-Hydroxytamoxifen (6 µg/0.1 mL/day, s.c.) effectively reduces methamphetamine-induced depletion of striatal dopamine in both intact and adrenalectomized male and female C57BL/6J mice without altering dopamine levels in the striatum[6].
References:
[1] Jordan VC, et al. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977 Nov;75(2):305-16.
[2] Davis KM, et al. Small molecule-triggered Cas9 protein with improved genome-editing specificity. Nat Chem Biol. 2015 May;11(5):316-8.
[3]Shagufta,Irshad,Ahmad.Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives[J].European Journal of Medicinal Chemistry: Chimie Therapeutique, 2018, 143:515-531.
[4] JORDAN V C, KOCH R, LANGAN S, et al. Ligand interaction at the estrogen receptor to program antiestrogen action: a study with nonsteroidal compounds in vitro[J]. Endocrinology, 1988, 122(4): 1449-1454.
[5] Defriend D , Anderson E , Bell J ,et al.Effects of 4-hydroxytamoxifen and a novel pure antioestrogen (ICI 182780) on the clonogenic growth of human breast cancer cells in vitro[J].Br J Cancer, 1994, 70(2):204-211.
[6] Kuo YM, et al. 4-Hydroxytamoxifen attenuates methamphetamine-induced nigrostriatal dopaminergic toxicity in intact and gonadetomized mice. J Neurochem. 2003 Dec;87(6):1436-43.
Average Rating: 5 (Based on Reviews and 22 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















