Tosedostat (CHR2797) (Synonyms: CHR-2797) |
| カタログ番号GC10406 |
アミノペプチダーゼ阻害剤
Products are for research use only. Not for human use. We do not sell to patients.
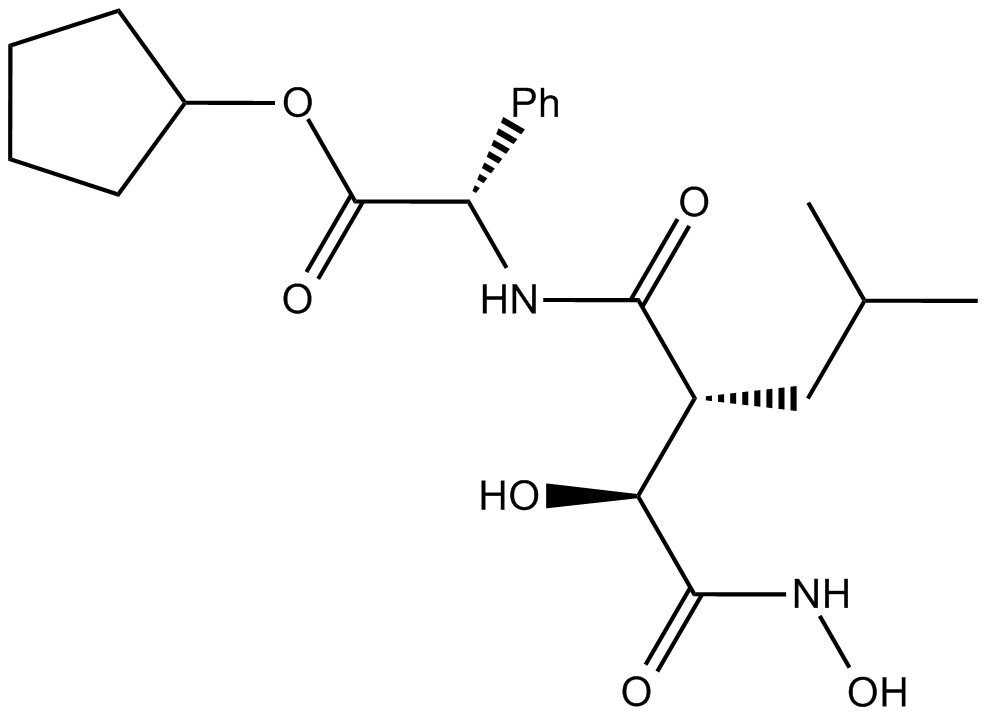
Cas No.: 238750-77-1
Sample solution is provided at 25 µL, 10mM.
Tosedostat is a novel and potent oral aminopeptidase inhibitor with clinical activity in a previous phase 1–2 study in elderly patients with relapsed or refractory acute myeloid leukaemia (AML). [2]
Aminopeptidases play a key role in the protein cell cycle. Inhibition of aminopeptidase results in the amino acid deprivation response, which occurs selectively in transformed cells and leads to upregulation of proapoptotic factors including CHOP and NOXA, activation of stress-related pathways such as NFκB, and inhibition of mTOR, which switches off protein synthesis. [2]
Tosedostat (CHR-2797) is converted intracellularly into a pharmacologically active metabolite CHR-79888. [1]
Tosedostat has antiproliferative, antiangiogenic and proapoptotic effects. Tosedostat is currently in a clinical trial phase for anticancer therapy, and displayed a broad antifungal activity against different Candida spp, including Candida glabrata. Tosedostat depletes sensitive tumour cells of amino acids by blocking protein recycling and thereby generates an antiproliferative effect. Tosedostat has activity in older patients with relapsed or refractory AML. [2]
References:
1.Van Herpen CM, Eskens FA, de Jonge M et al. A Phase Ib dose-escalation study to evaluate safety and tolerability of the addition of the aminopeptidase inhibitor tosedostat (CHR-2797) to paclitaxel in patients with advanced solid tumours. Br J Cancer. 2010 Oct 26;103(9):1362-8.
2.Cortes J, Feldman E, Yee K et al. Two dosing regimens of tosedostat in elderly patients with relapsed or refractory acute myeloid leukaemia (OPAL): a randomised open-label phase 2 study. Lancet Oncol. 2013 Apr;14(4):354-62.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















