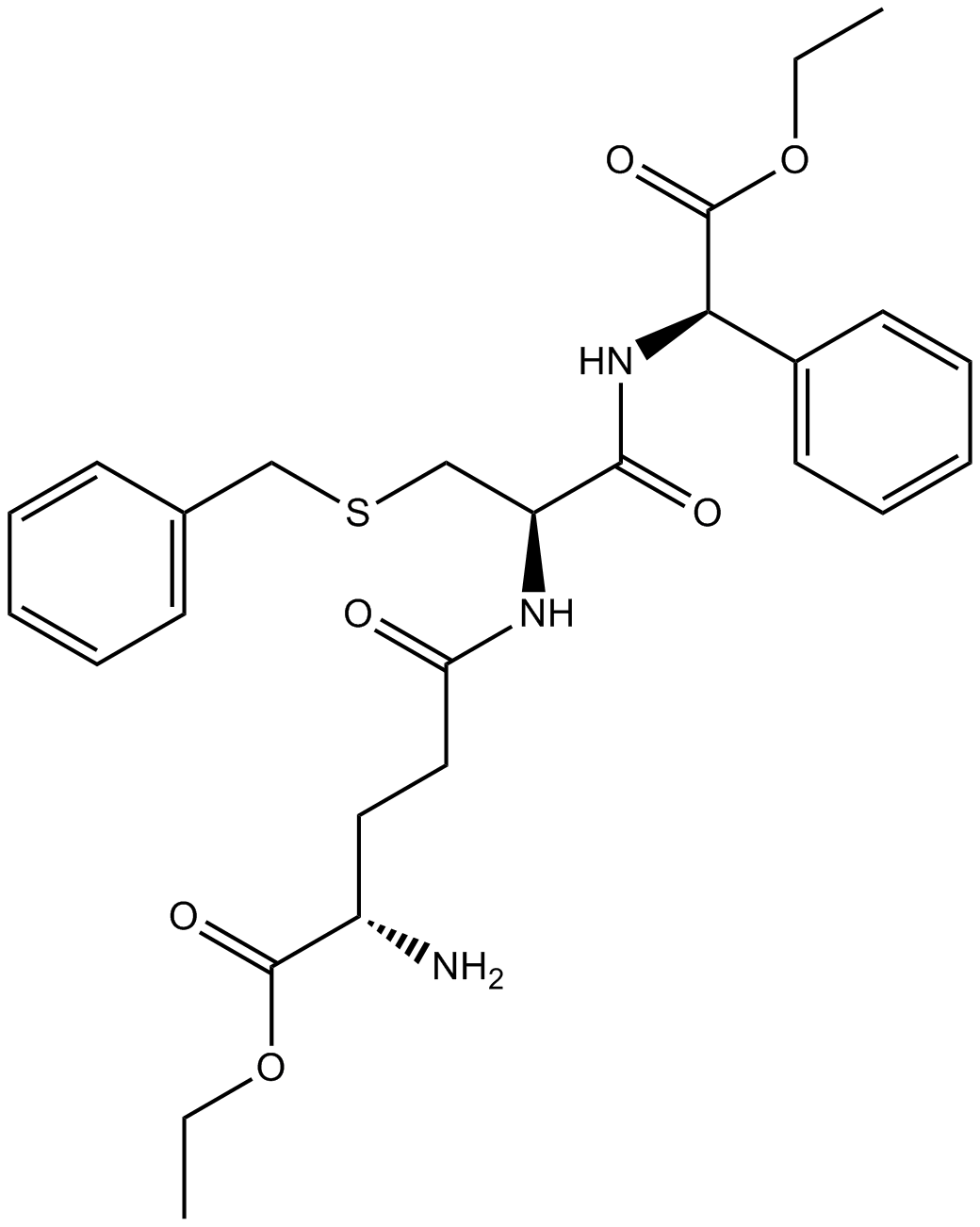
Ezatiostat (Synonyms: TER199;TLK199;Telintra) |
| カタログ番号GC18095 |
An inhibitor of GSTP1-1
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 168682-53-9
Sample solution is provided at 25 µL, 10mM.
Ezatiostat is a synthetic tripeptide analog of glutathione that inhibits the glutathione S-transferase P1-1 (GSTP1-1) [1].
Ezatiostat is a glutathione analog prodrug that has been shown to stimulate the proliferation of myeloid precursors. Ezatiostat is metabolized to TLK117 which shows selectivity to inhibit and bind to glutathione S-transferase P1-1 (GST P1-1). In addition, ezatiostat could activate the caspase-dependent pathway that help inhibit the emergence of malignant clones and may also contribute to apoptotic death and elimination by increasing reactive oxygen species (ROS) in dysplastic cells.
Ezatiostat has shown great stimulatory activity in vitro in human bone marrow progenitor cultures, as well as in several preclinical models of myelopoiesis in vivo. A multidose-escalation study of ezatiostat was performed for the treatment of myelodysplastic syndrome. Patients received 10 dose levels of ezatiostat tablets on days 1 to 7 of a 21-day cycle for a maximum of 8 cycles. Forty-five patients with low to intermediate-2 International Prognostic Scoring System risk myelodysplastic syndrome were enrolled. 17 hematologic improvement (HI) responses by International Working Group criteria were observed at dose range of 200 to 6000 mg/day , wherein 11 HI responses at dose range of 4000 to 6000 mg/day [1].
In randomized multicenter study of 2 extended dosing schedules of oral ezatiostat in low to intermediate-1 risk myelodysplastic syndrome. Patients were randomized by 1 stratification factor-baseline cytopenia to 1 of 2 extended dosing schedules. In general, 11 of 38 (29%) HI-Erythroid (HI-E) response was observed in patients who were red blood cell (RBC) transfusion-dependen. The median duration of HI-E response was 34 weeks. Ezatiostat is proven to cause clinically significant and sustained reduction in RBC transfusions, transfusion independence and multilineage responses in MDS patients [2].
References:
[1]. Raza A, Galili N, Smith S, et al. Phase 1 multicenter dose-escalation study of ezatiostat hydrochloride (TLK199 tablets), a novel glutathione analog prodrug, in patients with myelodysplastic syndrome. Blood, 2009, 113(26): 6533-6540.
[2]. Raza A, Galili N, Smith SE, et al. A phase 2 randomized multicenter study of 2 extended dosing schedules of oral ezatiostat in low to intermediate-1 risk myelodysplastic syndrome. Cancer, 2012, 118(8): 2138-2147.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















