Ciproxifan (Synonyms: FUB-359) |
| カタログ番号GC12924 |
シプロキシファン (FUB 359) は、9.2 nM の IC50 を持つ、ヒスタミン H3 受容体の強力で選択的な経口生物学的利用能および競合的アンタゴニストです。
Products are for research use only. Not for human use. We do not sell to patients.
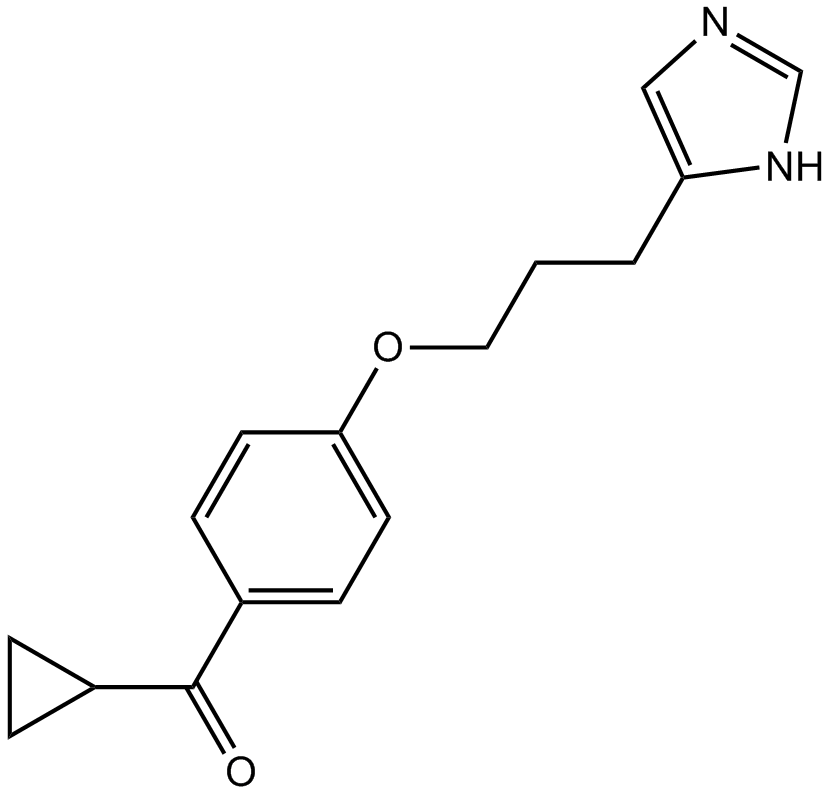
Cas No.: 184025-18-1
Sample solution is provided at 25 µL, 10mM.
Ciproxifan is a novel and potent antagonist of histamine H3-receptor with a IC50 value of 9.2±1.8nM [1].
Ciproxifan has shown the in-vitro antagonistic action to H3-receptor with a IC50 value of 9.2±1.8nM. In addition, Ciproxifan has been reported to competitively antagonize the (R) α-MeHA induced relaxation of electrically stimulated guinea pig ileum longitudinal muscle. Besides, Ciproxifan has been revealed to have the effect on [125I]iodoproxyfan binding with a Ki value of 0.7±0.2 nM. Apart from these, Ciproxifan has been found to be a selective antagonist with pKi values of 9.3, 4.9, 4.6, 5.5, 5.4, 4.9, <5.0, 4.8, <5.5 and <5.7 for H3, H2, H1, muscarinic M3, adrenergic α1D, β1, serotonin 5-HT1B, 5-HT2A, 5-HT3 and 5-HT4, respectively [1].
References:
[1] Ligneau X1, Lin J, Vanni-Mercier G, Jouvet M, Muir JL, Ganellin CR, Stark H, Elz S, Schunack W, Schwartz J. Neurochemical and behavioral effects of ciproxifan, a potent histamine H3-receptor antagonist. J Pharmacol Exp Ther. 1998 Nov; 287(2):658-66.
Average Rating: 5 (Based on Reviews and 40 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















