HOKU-81 |
| カタログ番号GC12243 |
Products are for research use only. Not for human use. We do not sell to patients.
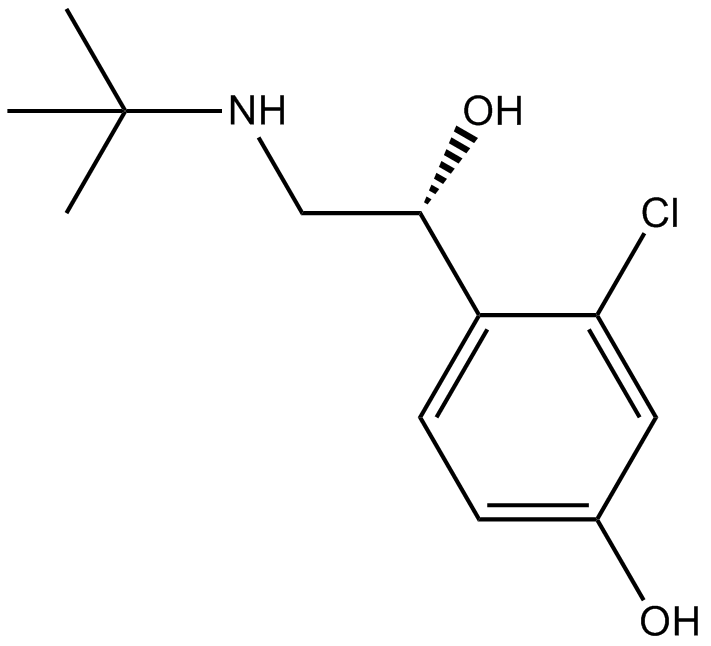
Cas No.: 58020-43-2
Sample solution is provided at 25 µL, 10mM.
HOKU-81, a new bronchodilator, is one of the metabolites of tulobuterol. IC50 Value: Target: Adrenergic ReceptorEffects of HOKU-81 on isolated trachea and atria of guinea pigs were compared with those of various bronchodilators. HOKU-81 appears to be a potent and selective beta 2-stimulant with a slight inotropic action. HOKU-81 was approximately 8 times more potent than tulobuterol, approximately twice as potent as salbutamol, and approximately as potent as isoprenaline in relaxing effect on the isolated tracheal smooth muscle preparation of guinea pigs. This effect of HOKU-81 seems to be due to direct action on the adrenergic beta-receptor.
References:
[1]. Kubo S, Matsubara I, Yamazaki M et al. Pharmacological studies of 1-(2-chloro-4-hydroxyphenyl)-2-t-butylaminoethanol (HOKU-81), a new bronchodilator. 1st Communication: Bronchodilator and cardiovascular actions. Arzneimittelforschung. 1980;30(8):1272-8.
[2]. Gomi Y, Shirahase H, Funato H. Effects of 1-(2-chloro-4-hydroxyphenyl)-t-butylaminoethanol (HOKU-81), a new bronchodilator, on isolated trachea and atria of guinea pig. Jpn J Pharmacol. 1979 Aug;29(4):515-24.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















