MLN3126 |
| カタログ番号GC65990 |
MLN3126 は、経口で有効な強力な CCR9 拮抗薬です。 MLN3126 は、マウス初代胸腺細胞の CCL25 誘導性カルシウム動員および走化性を阻害し、カルシウム流入に対する IC50 値は 6.3 nM です。
Products are for research use only. Not for human use. We do not sell to patients.
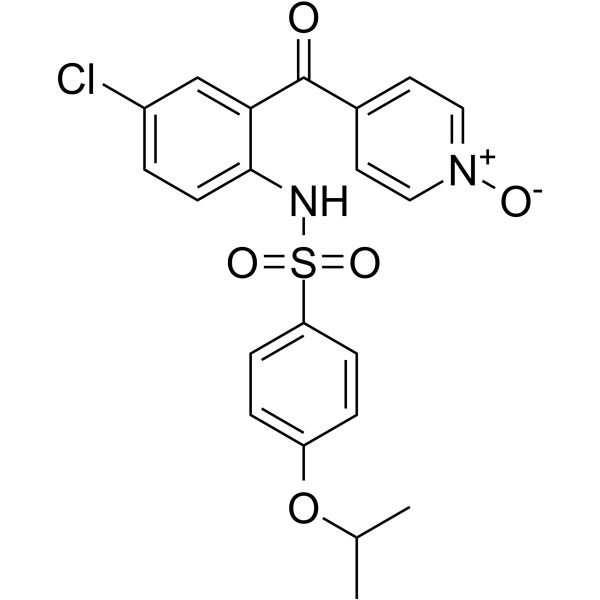
Cas No.: 628300-71-0
Sample solution is provided at 25 µL, 10mM.
MLN3126 is an orally active and potent CCR9 antagonist. MLN3126 inhibits CCL25-induced calcium mobilization and chemotaxis of mouse primary thymocytes, wiht an IC50 value of 6.3 nM for calcium influx[1].
MLN3126 inhibits CCL25-induced calcium mobilization with an IC50 value of 6.3 nM in CCR9 expressing cells[1].
MLN3126 inhibits the binding of biotinylated CCL25 to CCR9 with an IC50 of 14.2 nM[1].
Cell Invasion Assay[1]
| Cell Line: | Mouse thymocytes |
| Concentration: | 0.01, 0.03, 0.1, 0.3, 1, 3 μM |
| Incubation Time: | 90 min |
| Result: | Inhibited CCL25-induced chemotaxis of mouse thymocytes. |
MLN3126 (2.5% w/w; p.o.) decreases colonic level of IFN-γ, largely produced by T cells[1].
MLN3126 (0.05, 0.25 and 1% (w/w); p.o.) has the potential activity for alleviating inflammatory bowel disease (IBD)[1].
| Animal Model: | Activated T cell transferred colitis mouse model[1] |
| Dosage: | 0.05, 0.25 and 1% (w/w) (around 4 g/day) |
| Administration: | Oral gavage; 21 days |
| Result: | Blocked CCR9/CCL25 interaction by inhibiting migration of T cells to the colon and resulted in the amelioration of colitis. |
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















