Roluperidone (CYR-101) (Synonyms: CYR-101; MIN-101; MT-210) |
| カタログ番号GC30206 |
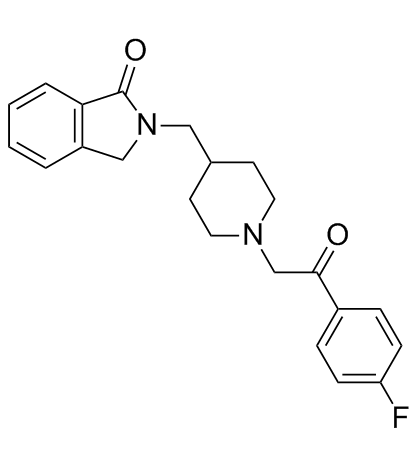
ロルペリドン (CYR-101) (CYR-101) は、5-HT2A およびシグマ-2 受容体に対して高い等効力の親和性を持つ新規の環状アミド誘導体です (5-HT2A およびシグマ-2 に対する Ki は、それぞれ 7.53 nM および 8.19 nM)。 .
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 359625-79-9
Sample solution is provided at 25 µL, 10mM.
Roluperidone (CYR-101) is a novel cyclic amide derivative that has high equipotent affinities for 5-HT2A and sigma-2 receptors (Ki of 7.53 nM and 8.19 nM for 5-HT2A and sigma-2, respectively).
Roluperidone (CYR-101) also shows binding affinity for α1-adrenergic receptors but low or no affinity for muscarinic, cholinergic, and histaminergic receptors. Although Roluperidone (CYR-101) has no affinities for pre- or postsynaptic dopaminergic receptors, it is probable that sigma-2 receptors are implicated in the modulation of dopamine and glutamatergic pathways, as well as in calcium neuronal modulation[1].
[1]. Davidson M, et al. Efficacy and Safety of MIN-101: A 12-Week Randomized, Double-Blind, Placebo-Controlled Trial of a New Drug in Development for the Treatment of Negative Symptoms in Schizophrenia. Am J Psychiatry. 2017 Jul 28:appiajp201717010122.
Average Rating: 5 (Based on Reviews and 35 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















