Azvudine hydrochloride (Synonyms: RO-0622 hydrochloride; FNC hydrochloride) |
| Catalog No.GC60071 |
Azvudine(RO-0622) 염산염은 HIV, HBV 및 HCV에 대한 항바이러스 활성이 있는 강력한 뉴클레오시드 역전사효소 억제제(NRTI)입니다.
Products are for research use only. Not for human use. We do not sell to patients.
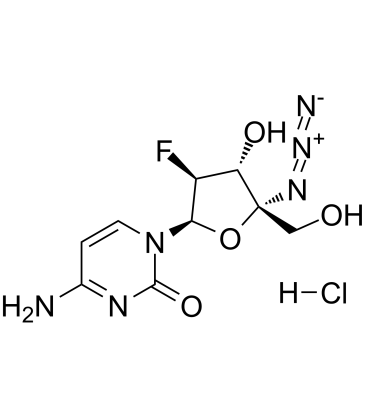
Cas No.: 1333126-31-0
Sample solution is provided at 25 µL, 10mM.
Azvudine hydrochloride is a potent nucleoside reverse transcriptase inhibitor (NRTI) that has antiviral activity against HIV, HBV, and HCV,(HIV-1 (EC50s 0.03 to 6.92 nM) and HIV-2 (EC50s 0.018 to 0.025 nM))[1]. Azivudine hydrochloride could inhibit SARS-CoV-2 and HCoV-OC43 coronavirus with EC50 1.2-4.3 µM[3].
In C8166 cells,Azvudine hydrochloride showed strong inhibition against wild-type HIV-1 IIIB and HIV-1 RF, with 50% effective concentration values (EC 50) ranging from 30 to 110 pM[2].
EV71 and CA16 challenge resulted in 90% and 30% mortality, respectively, while Azvudine hydrochloride treatment greatly reduced mortality to 20% and 0%. Azvudine hydrochloride treatment significantly improved clinical presentation and survival, indicating that Azvudine hydrochloride effectively protects against EV71 and CA16 challenge in vivo[4].
Treating SARS-CoV-2 infected rhesus macaques with Azvudine hydrochloride (0.07 mg/kg, qd, orally) reduced viral load, recuperated the thymus, improved lymphocyte profiles, alleviated inflammation and organ damage, and lessened ground-glass opacities in chest X-ray[3].
Azvudine hydrochloride clinical efficacy in curing COVID-19 was significant, showing inhibition of SARS-CoV-2 replication in all 31 patients after treatment with Azvudine hydrochloride, and the anti-coronavirus activity of Azvudine hydrochloride was also demonstrated in animal experiments using RM[5].
References:
[1]: Wang RR, Yang QH, et,al. Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PLoS One. 2014 Aug 21;9(8):e105617. doi: 10.1371/journal.pone.0105617. PMID: 25144636; PMCID: PMC4140803.
[2]: Zhou Y, Zhang Y, et,al.Novel nucleoside analogue FNC is effective against both wild-type and lamivudine-resistant HBV clinical isolates. Antivir Ther. 2012;17(8):1593-9. doi: 10.3851/IMP2292. Epub 2012 Aug 9. PMID: 22910281.
[3]: Zhang JL, Li YH, et,al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct Target Ther. 2021 Dec 6;6(1):414. doi: 10.1038/s41392-021-00835-6. PMID: 34873151; PMCID: PMC8646019.
[4]: Xu N, Yang J, et,al.The Pyrimidine Analog FNC Potently Inhibits the Replication of Multiple Enteroviruses. J Virol. 2020 Apr 16;94(9):e00204-20. doi: 10.1128/JVI.00204-20. PMID: 32075935; PMCID: PMC7163137.
[5]: Ren Z, Luo H, et,al. A Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study. Adv Sci (Weinh). 2020 Aug 13;7(19):2001435. doi: 10.1002/advs.202001435. PMID: 32837847; PMCID: PMC7404576.
[6]: Fayzullina D, Kharwar RK, et,al. FNC: An Advanced Anticancer Therapeutic or Just an Underdog? Front Oncol. 2022 Feb 10;12:820647. doi: 10.3389/fonc.2022.820647. PMID: 35223502; PMCID: PMC8867032.
Average Rating: 5 (Based on Reviews and 29 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















