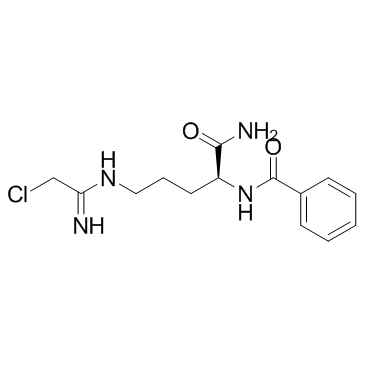
Cl-Amidine
Chemically, N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide is the full name of Cl-Amidine. The Cl-Amidine molecule is small, i.e., low atomicity and thus low molecular weight. As its name suggests it is an amidine having a halo atom, i.e., chlorine. The two-dimensional view of the molecular structure of the Cl-Amidine compound is diagrammatically presented in Figure 1.
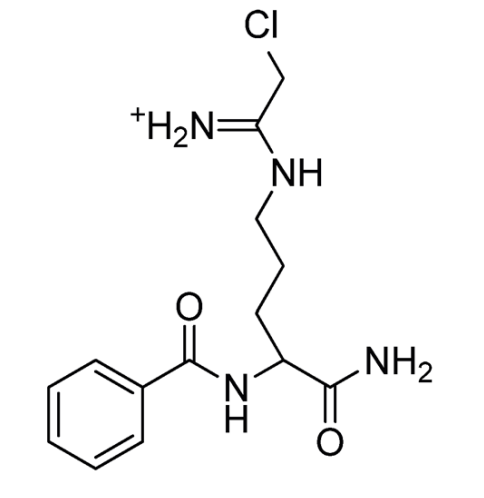
Figure 1 The two-dimensional view of the molecular structure of the Cl-Amidine compound
The Cl-Amidine inhibits all the proteins belonging to the protein arginine deiminase (PAD) family; thus, Cl-Amidine is termed as pan-PAD inhibitor. This is marked by the formation of a covalent bond in an irreversible reaction.
The protein arginine deiminase (PAD) family contains five distinct proteins namely PAD1, PAD2, PAD3, PAD4, and PAD6. All these proteins are the enzymes and thus catalyzes the citrullination/deamination reaction. Citrullination (aka deamination) is post-translational modification in which citrulline amino acid is chemically added to the target protein while at the same time the amino group of the arginine amino acid in the target protein is chemically removed. Moreover, citrullination (aka deamination) being a hydrolytic reaction involves a water molecule and also is dependent on the availability of calcium ions. A general overview of the citrullination (aka deamination) is demonstrated in Figure 2.
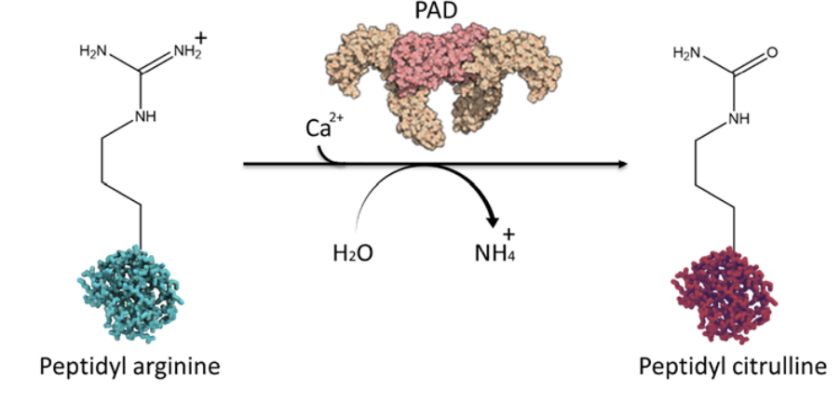
Figure 2 An overview of the citrullination (aka deamination) reaction. The intake of calcium ion and water molecule is shown. Also, the removal of amino group in the form of ammonium is indicated along with the addition of citrulline amino group in the target protein. In this biochemical reaction, calcium ion plays the role of activator. Actually, the amount of calcium ions increases, these ions interact with the protein arginine deiminase (PAD) proteins and causes some conformational changes in them. As a result of these changes, active site is formed and thus all PAD proteins become active and cause the citrullination (aka deamination) reaction.
There are two proposed mechanisms for the citrullination (aka deamination) reaction. One mechanism is a single step while the other one is a multistep mechanism. Both these mechanisms are outlined in Figure 3 and Figure 4, respectively.
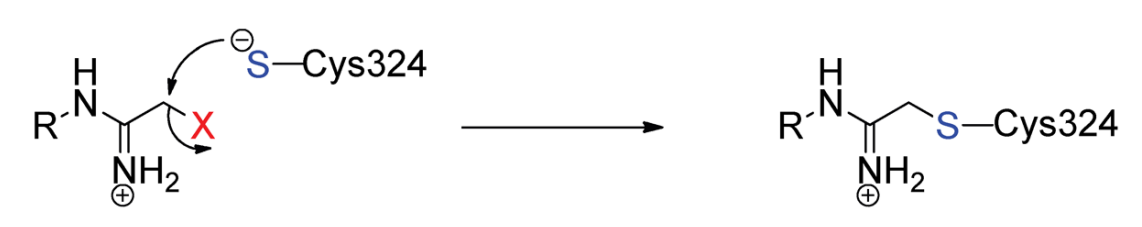
Figure 3 The Proposed Mechanism of the citrullination (aka deamination) reaction. This is a single step mechanism; thus, it is simple.
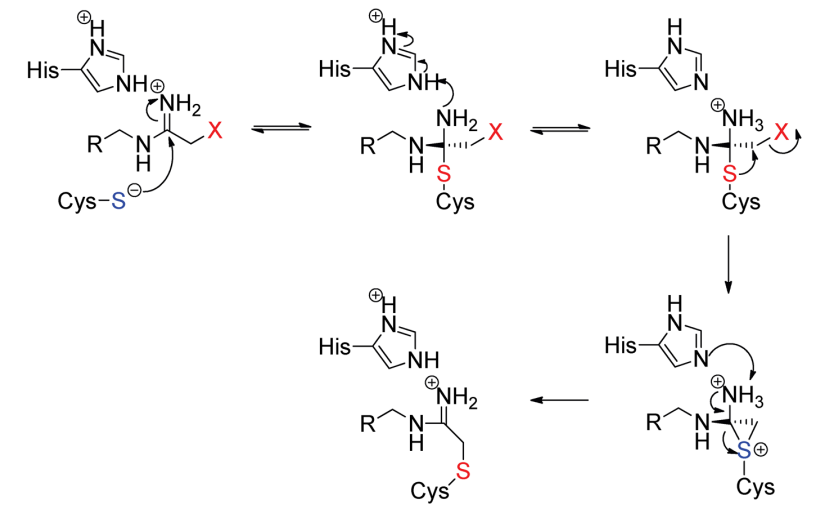
Figure 4 Another Proposed Mechanism for the citrullination (aka deamination). This is a multi-step mechanism and a little complex as well.
As stated earlier, the citrullination (aka deamination) is a post translational modification; thus, there exists a broad spectrum of the target proteins including cytoplasmic, nuclear, mitochondrial ones and the proteins of cytoskeleton. Histones and fibrinogen are the major target proteins. As a result of citrullination (aka deamination), a lot changes happen in a target protein including its structural folding, functional activity, isoelectric point (Wang et al.), Mobility in the electrophoresis, and antigenicity.
In certain anormal pathological conditions, for example lupus, inflammatory bowel disease (IBD), rheumatoid arthritis (Willis et al.), cancer, diabetes, and neurological disorders including Alzheimer's disease (AD), multiple sclerosis (MS) which are marked by the inflammation; it is common to them that PAD proteins are abnormally expressed in greater amount. On the molecular level, increased levels of the proinflammatory cytokines, Tumor Necrosis Factor alpha (TNF-α) and chemokines (IL-8), intracellular Ca2+, and ROS like superoxide ion and H2O2 contribute to the overexpression of PAD proteins.
The PAD4 protein, that is one of the targets of Cl-Amidine, contains a nuclear localization signal and thus, it gets localized in the nucleus of the oligodendrocytes during inflammation. There, it brings about citrullination (aka deamination) of the histone H3 protein molecules that eventually leads to the apoptosis in those cells which is a type of programmed cell death. Moreover, PAD4 protein also promotes breast cancer as it interferes with the target genes of oestrogen receptor. The same citrullination reaction is actually involved in the formation of Neutrophil Extracellular Trap (NETs) by the neutrophils when the target proteins are histone. In the formation of NETs, a fibrillar matrix is formed that is made up of decondensed chromatin in the extracellular space that eventually leads to the death of the surrounding pathogenic cells. This phenomenon of cell death is termed as NETosis, an overview of the NETs formation is depicted in Figure 5.
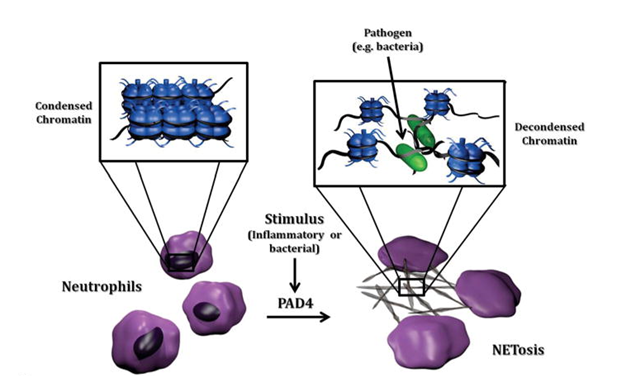
Figure 5 The formation of NETs by the Neutrophils
The formation of NETs by the infiltered neutrophils and pathogens specifically bacteria (due to the disrupted intestinal lining), initiates the inflammation vis the formation of proinflammatory cytokines including Tumor Necrosis Factor alpha (TNF-α), Interleukin 1 beta (IL-1β), Interleukin 6 (IL-6) and Interleukin 7 (IL-7) that eventually leads to the colitis. This is summarized in Figure 6 (Wang et al., 2023). Whereas, in the blood vessels the NETs formed by the neutrophils could lead to formation of immunothrombus as shown in Figure 7.
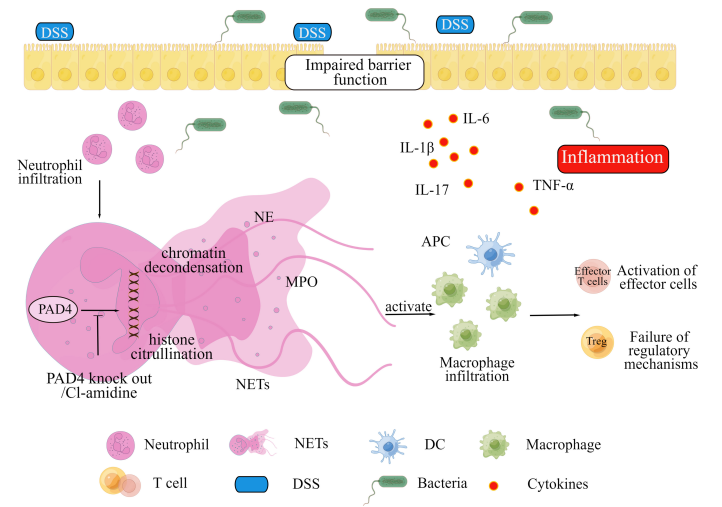
Figure 6 The formation of NETs in the Colitis, is mediated by the activity of PAD4. This activity can be blocked by the Cl-Amidine treatment
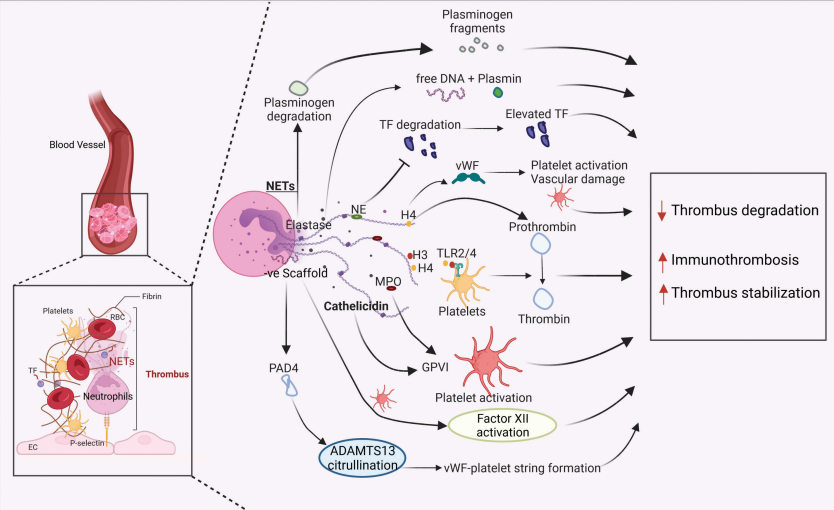
Figure 7 The formation of NETs by the neutrophils, leading to immunothrombus formation
On the molecular level, the inhibition of the PAD proteins is marked by the suppressed expression of interleukin 6 (IL-6), interleukin 17 (IL-17) and Tumor necrosis factor alpha (TNF-α).
There are several central nervous system pathologies which are marked by the hyperactivity of the PAD proteins. In these pathologies, the Kyoto Encyclopedia of Genes And Genomes (KEGG) pathways are regulated and thereby a number of molecular events are initiated within the central nervous system. For example, the protein moonlighting (the acquisition of many biological functions by a single peptide), release of extracellular vesicles, generation of neoepitopes occur, disrupted mitochondrial functions and gene regulation. These molecular events lead to the structural damage to the neural tissues in the form of injuries, degenerative diseases and the neoplasms of the central nervous system and, are summarized in Figure 8.
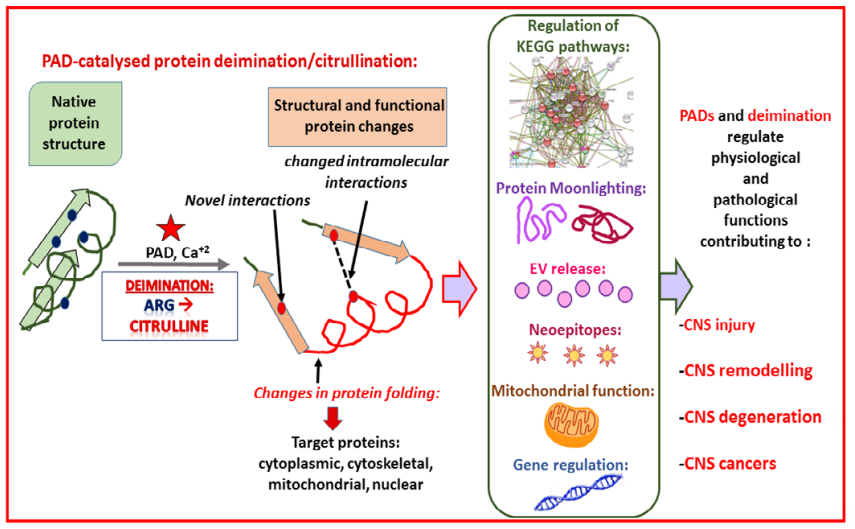
Figure 8 The negative role of protein arginine deiminase (PAD) proteins due to their hyperactivity leading the central nervous system pathologies
In the animal model specifically in the murine model of collagen-induced arthritis (CIA), the clinically significant findings have been observed regarding the treatment of rheumatoid arthritis (Willis et al.) via the Cl-Amidine. In the in vitro laboratory settings, the inhibition of PAD1, PAD2, PAD3 and PAD4 via Cl-Amidine has been demonstrated; for PAD6 it could be demonstrated as PAD6 does not demonstrate its enzymatic activity in the in vitro settings. The Cl-amidine treatment has been found to reduce the overall disease severity in terms of inflammation, pannus and damage to bone and cartilage (as graphically shown in Figure 9) as well as the joint damage at synovium and cartilage (as graphically shown in Figure 10) in a dose-dependent manner (as graphically shown in Figure 11)
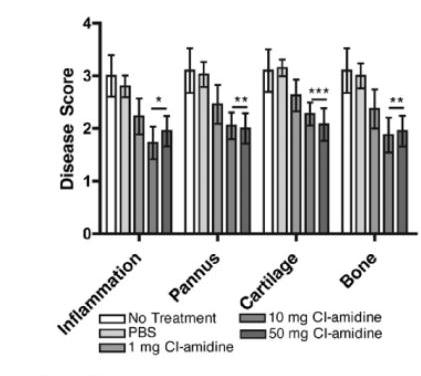
Figure 9 The Cl-amidine treatment causes a decrease in the severity of collagen-induced arthritis (CIA) in the murine model
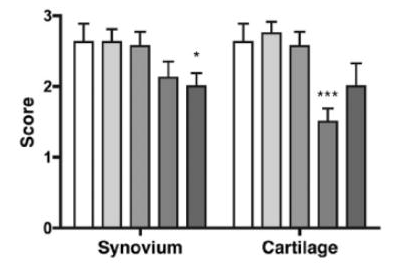
Figure 10 The Cl-amidine treatment causes a decrease in the joint damage in the murine model of collagen-induced arthritis (CIA). (The legends are same as for Figure 7)
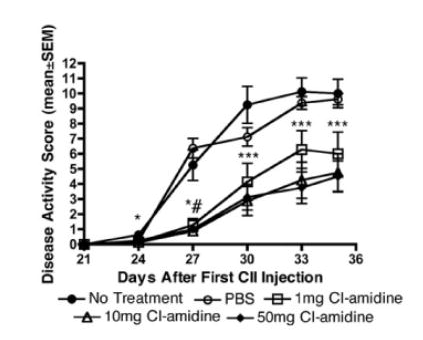
Figure 11 The treatment decreases the severity of collagen-induced arthritis (CIA) in the murine model a dose-dependent manner












Comments