Clozapine N-Oxide
The substance clozapine N-oxide (CNO) has recently come to light due to its application in pharmacology and neuroscience studies. Some of the uses of clozapine N-oxide may include inhibition of short-term memory recovery in mice expressing hM4Di in the hippocampal neurones or silencing them in vitro, preventing locomotion in mice expressing hM3Dq in GABA neurones in the ventral tegmental area and so on. CNO is a metabolite of the antipsychotic drug clozapine, frequently prescribed to treat schizophrenia, derived from human muscarinic acetylcholine receptors and is an activator of DREADDs (Designer Receptors Exclusively Activated by Designer Drugs). Neuroscience researchers are increasingly using DREADDs, which are altered G-protein-coupled receptors (GPCRs) having lost the affinity for the original ligand they are triggered by a specially designed actuator. It is a group of designed human muscarinic receptors that only react to the artificially created ligand, that is CNO. DREADDs is a chemo-genetic approach that is frequently utilised to control neural processes in animals that are free to move around. They appear to be chemically inactive in mice and rats which were designed to respond to nanomolar (nM) doses of CNO.
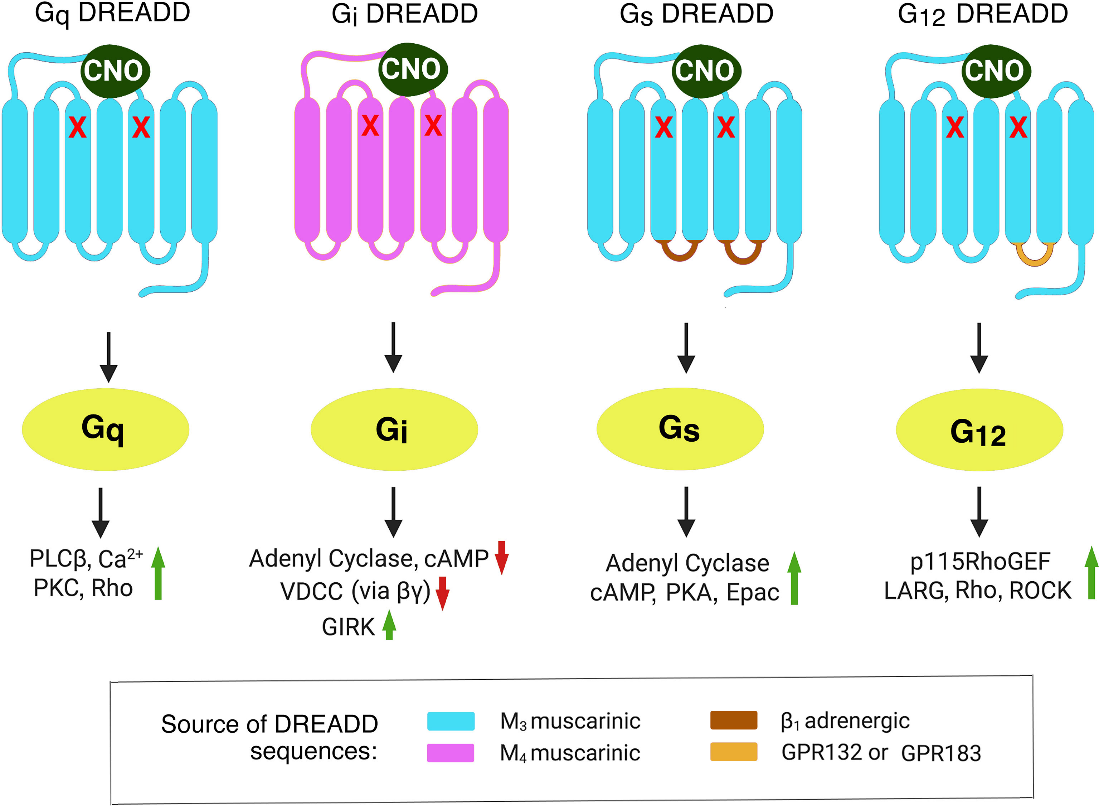
Figure 1: The DREADDs displayed are all highly potent and effective CNO-activatable mutant muscarinic acetylcholine receptors.
The DREADD technology is based on the use of "designer receptors" (the mutated human muscarinic receptors; hM3Dq and hM4Di) that are not activated by other endogenous neurotransmitters but are only stimulated via "designer drug", the CNO (a passive and inert clozapine metabolite). DREADDs allow for the non-invasive, minute- to hour-scale remote regulation of neural activity. Due to its special features, clozapine N-oxide is used as an instrument in experiments even though it has little clinical application as a medicinal drug. DREADDs, as opposed to opsins, are utilised to stimulate or inhibit particular neurons. The stimulating DREADD, hM3Dq is a modification of the human M3 muscarinic receptor and exhibits a low binding for acetylcholine, its natural ligand. However, shows a high binding for the CNO, which is synthetic. Targeted neurons fire in bursts once this Gq-coupled receptor gets stimulated by local or systemic injection of CNO. Alternatively, targeted neurons are frequently silenced with CNO by the Gi-coupled hM4Di DREADD, another modified human M4 muscarinic receptor. CNO thus triggers a downstream signalling cascade causing the targeted neurons to either burst fire (for hM3Dq) or be silenced (for hM4Di), allowing for sustained neuronal excitement or inhibition.
According to Jendryka et al., the effects of injecting CNO, intraperitoneally, in mice tend to be noticeable in 10-15 minutes, peak in 45–50 minutes and then gradually return to baseline after about 9 hours. For mice, researchers commonly inject 0.1-3 mg/kg of CNO intraperitoneally, while intracranial microinjections have also been employed. CNO is soluble in aqueous solutions like normal saline.
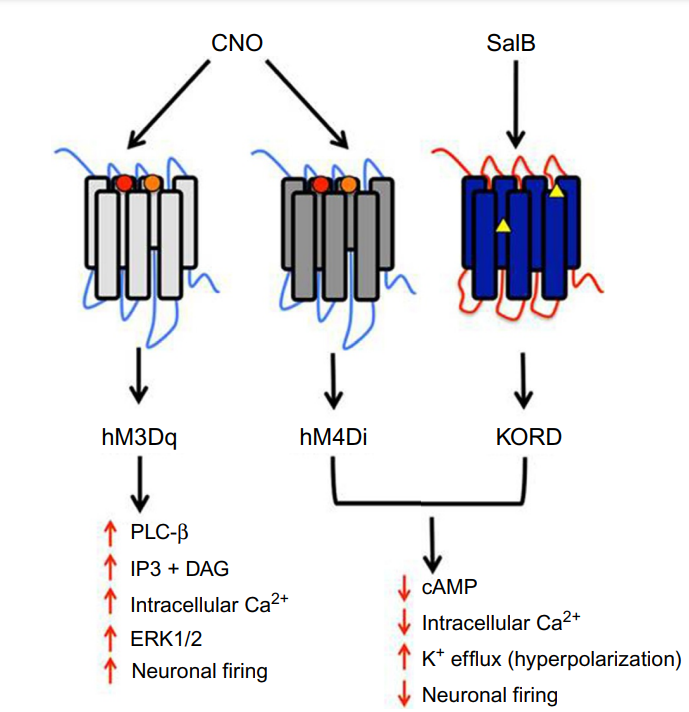
Figure 3: The functioning of the two synthetic agents Salvinorin B (SalB) and Clozapine-N-oxide (CNO).
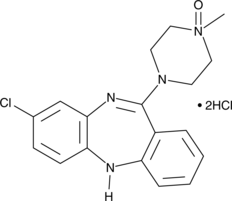
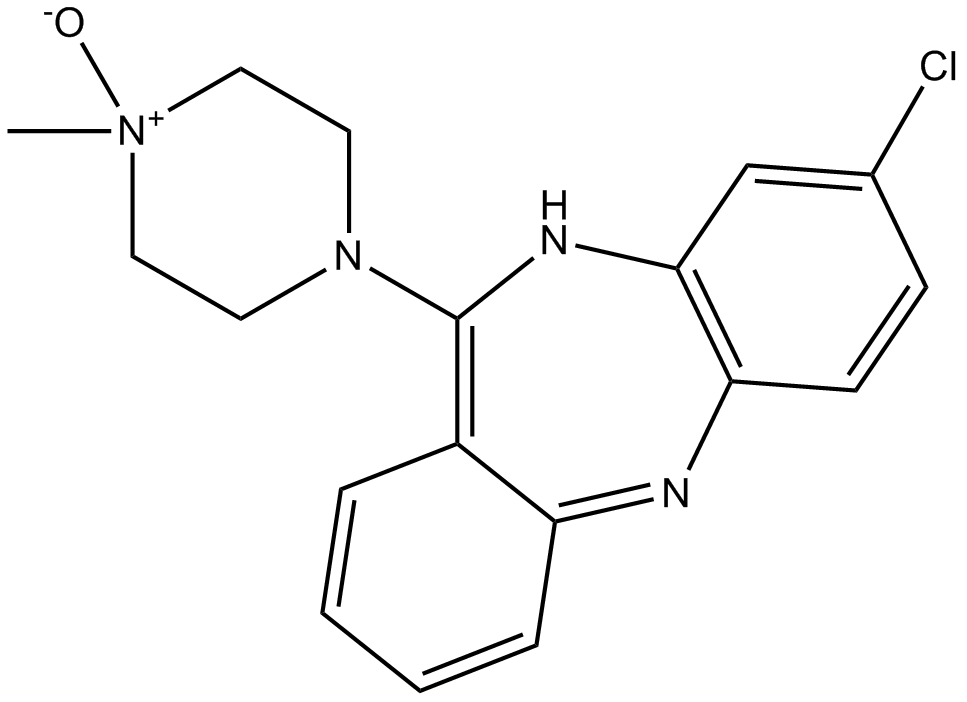
Figure 1 shows the function of Salvinorin B (SalB): activates kappa opioid receptor (KOR)-based DREADDs, and Clozapine-N-oxide (CNO): activates human muscarinic (hM)-based DREADDs. CNO inhibits hM4Di-expressing neurons and induces burst firing in those expressing hM3Dq. While SalB blocks KORD-expressing neurons but enables bidirectional chemogenetics in ones expressing both hM3Dq and KORD. However, it was recently discovered that clozapine, a CNO derivative that can pass the blood-brain barrier, actually activates the hM3Dq and hM4Di DREADDs instead of CNO. Following systemic medication injections, CNO cannot pass through the brain and has a weak affinity for DREADDs. Some studies revealed that in mice, CNO is pharmacologically inactive and does not undergo back-metabolization to its parent chemical form, clozapine. On the contrary recent investigations have shown that CNO can be effectively converted, in vivo, to metabolites that are pharmacologically active like clozapine. Clozapine belongs to the chemical group of benzisoxazole derivatives and has a low molecular weight (MW = 326.13). The chemical name is 8-chloro-11-(4-methyl-4-oxido-1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepine and it has a molecular formula C18H19ClN4O. It is a tricyclic benzodiazepine with three H-bond acceptors and a single H-bond donor.
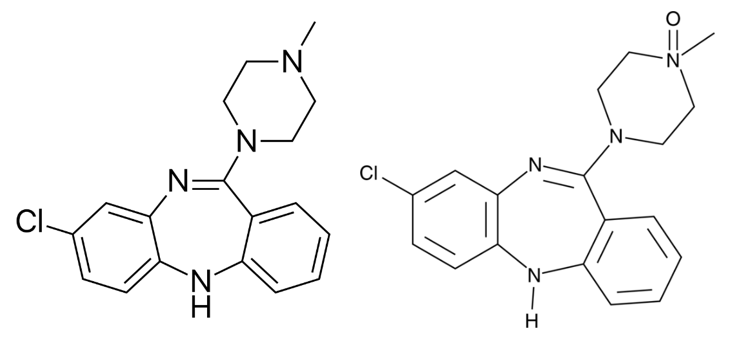
Figure 4: The 2D Molecular Structures of Clozapine (left) and Clozapine N-Oxide (right).
Clozapine has a strong affinity and efficacy for DREADDs, dopamine D2 and serotonin 5-HT2A receptors. Its remarkable efficacy relative to other antipsychotics may be due to its diverse interaction pattern with other receptors like cholinergic, histaminergic and adrenergic. When injected in vivo CNO converts to clozapine which rapidly enters to brain occupying CNS-expressed DREADDs. Gomez et al. revealed that CNO has very little CNS presence and has unfavourable results in vivo and in vitro pharmacology towards activating DREADD. As clozapine is pharmacologically very active at different sites it is critical to measure its concentration in the plasma and cerebrospinal fluid (CSF) shortly after CNO injection. Due to the quick conversion of CNO into clozapine, which was first seen in guinea pigs and humans and later in rodents, there may be detectable quantities of clozapine within the bloodstream and CSF.
An experimental study by Manvich et al., shed light on the conversion of CNO to clozapine and its potential for clozapine-like effects in ordinary laboratory rats and mice. It stated that in both rats and mice, CNO is certainly reverse-metabolized to clozapine, albeit a different mouse study revealed that the amount of clozapine present after CNO injection was negligible. When administered a 1.25 mg/kg test dose of clozapine or 10.0 mg/kg of CNO (doses enough to activate DREADDs) it produced moderate levels of clozapine substitution in rats while high levels in mice. The mean concentration values of clozapine, CNO, and N-desmethylclozapine in plasma were recorded at 30 and then 60-minute intervals, after injecting 10.0 mg/kg CNO or 1.25 mg/kg clozapine as shown in Figure 3.
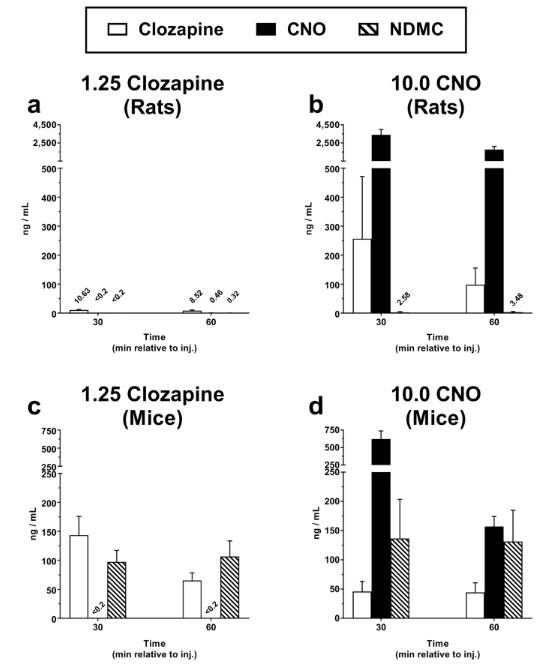
Figure 5: Graphs show the pharmacokinetic analysis of clozapine in rats (a) and mice (c) and of CNO in rats (b) and mice (d).
Clozapine is quickly metabolised to NDMC (metabolite N-desmethylclozapine) in mice but slowly metabolised to CNO and NDMC in rats these outcomes may help explain why different species have different reactions to CNO. However, it is crucial to recognise interspecies variations in how converted clozapine behaves.
The recognised conversion of CNO to clozapine is considered a constraint for the therapeutic usage of DREADDs, although its use has the potential to have negative side effects, it has already received approval from the American Food and Drug Administration. Clinically clozapine has long been employed therefore, it is said to have well-established pharmacokinetic, pharmacodynamic and adverse effect profiles. However, researchers employing DREADD technology should be using low levels of clozapine instead of large amounts of CNO. It would further be helpful to find other CNO analogues with strong drug-like characteristics, outstanding CNS penetrability and desirable pharmacological and neurotoxic aspects to develop new small molecule actuators. One of these analogues, or a future chemical, might not be converted into substances similar to clozapine and lesser off-targets.













Comments