Phos-tag Acrylamide
A useful chemical called Phos-tagTM is capable of capturing phosphorylated His/Asp/Lys and Ser/Thr/Tyr. It is a synthetic chemical molecule that was created using the catalytic domain of alkaline phosphatase as a model. Phos-tagged proteins can be separated, detected, analyzed using mass spectrometry, and purified using a range of procedures that use Phos-tagTM. An innovative and useful method for the enrichment and analysis of phosphorylated proteins is offered by phospho-tag acrylamide, a customized reagent that is specifically made to bind phosphorylated proteins during electrophoresis.
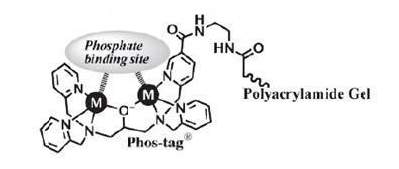
Figure 1: The structure of Phos-tag Acrylamide.
An alternative to conventional acrylamide gel electrophoresis is phospho-tag acrylamide. It has a phosphate-binding component that interacts with phosphorylated residues on proteins only—usually a metal chelate. Phos-tag acrylamide's metal ions create coordination interactions with phosphate groups, which causes phosphorylated proteins in the gel matrix to lag. During migration, phosphorylated proteins are captured by divalent metal ions. The migratory velocity decreases with increasing phosphorylation. Phosphorylation levels provide the basis for separation (Figure2). (Even at the same levels of phosphorylation, separation may still happen if the phosphorylation sites differ)
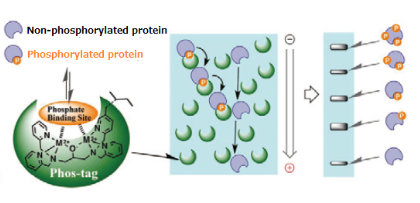
Figure 2: Principle of phosphorylation via phos-tag.
Phos-tag, a novel zinc compound that binds phosphate, was initially reported in 2003. This was subsequently evolved into acrylamide-dependent Phos-tag for inclusion in SDS-PAGE and biotin-pendant Phos-tag for labeling of phosphorylated proteins in gels and blots. Phos-tag acrylamide and Mn2+ ions were simply added to the separating gel in the initial iteration of Phos-tag gels. The alkaline conditions of normal SDS-PAGE hindered phosphate binding, hence Zn2+-ions were initially unusable. Casein was one of the first phosphorylated proteins examined in the groundbreaking study by Kinoshita et al. Dephosphorylated casein ran as a single band on Phos-tag gels, while β-casein produced eight distinct bands that each corresponded to a different phospho-site pattern (Figure 3). It's noteworthy to note that the phospho-versions of casein exhibit a little upward shift in the lane without Phos-tag (Fig. 3B). This is an illustration of how phosphorylation can occasionally cause an alteration in apparent molecular weight in conventional SDS-PAGE. Significantly, Kinoshita et al. may demonstrate the simultaneous detection of phosphorylated and dephosphorylated forms of a protein. Phos-tag gels were successfully tested for detachment of phosphorylated proteins from the Phos-tag in the gel by soaking the gels in EDTA solution beforehand to eliminate excess Mn2+-ions.
Numerous Phos-tag gel electrophoresis advancements were published after the publication of this original work. The significant finding that Phos-tag SDS-PAGE can distinguish between phosphoprotein species with the same amount of phosphate groups but on distinct locations was made in 2008 . These findings suggested that the total amount of incorporated phosphates is not the only factor influencing the changed mobility of phosphorylated proteins in Phos-tag gels. Rather, amino acid residues around the phospho-site seem to affect SDS binding, Phos-tag, or both, and hence running patterns . For instance, it has been demonstrated that proteins on Phos-tag gels are slower to titrate when their glutamate residues are present. It is believed that the negative charge of glutamate's carboxylate group resembles the negative charge of phosphate, which causes Phos-tag binding and a slower protein passage through the gel. Proteins phosphorylated on histidine residues in addition to serine, threonine, and tyrosine can be separated using phospho-tag gels .
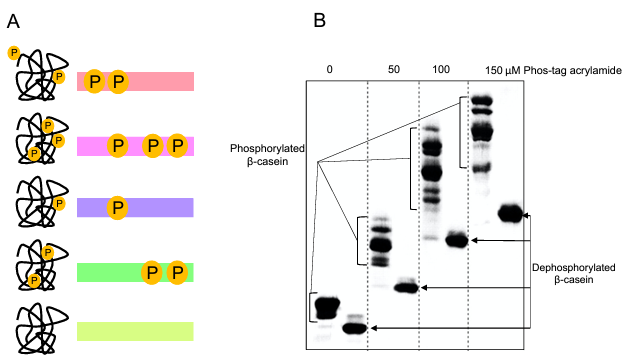
Figure 3: Phos-tag gel electrophoresis. A. Schematic overview of the Phos-tag gel method. B. Phos-tag gel separation of β-casein.
Based on a neutral pH gel system, Zn2 -Phos-tag gel electrophoresis was introduced in 2011 . This method permits the use of Zn2+-ions for Phos-tag interactions. It was demonstrated that this approach outperformed the Mn2+method in terms of phosphorylated protein separation. Numerous reviews and method papers detailing different facets of Mn2+ and Zn2+-based Phos-tag gel electrophoresis have been published in recent years. Multiple phospho-sites of any protein can be simultaneously detected and quantified by phospho-tag gel electrophoresis, Coomassie staining, or Western blotting. An examination of MEK1, a crucial kinase in the mitogen-activated kinase pathway that is located downstream of growth factor receptors, provides a good illustration. The phosphorylation of MEK1 and alterations in phosphorylation patterns after EGF treatment were discussed by the authors in this work. Four MEK1 phospho-sites in all were concurrently found and measured in the basal state, both as single sites and in different multi-site combinations. Using phospho-specific antibodies and mutagenesis, the phosphorylations underlying each band were verified. Running patterns of protein species that were phosphorylated were not just determined by the quantity of phosphates; rather, they were also influenced by the phosphates' location within the protein and, potentially, by residues close to the phospho-site (Figure 4). For instance, band movement was slower when phosphorylation of a single site (Fig. 4A, S298, band #5) was paired with phosphorylation of three other sites (Fig. 4, S292/T386/T388, band #4). After cells were treated with epidermal growth factor (EGF), endogenous MEK1phosphorylation produced two additional bands, #8 and #9, which were found to be associated with phosphorylation of the primary MEK1 activation sites, S218 and S222 (Fig. 4, indicated in yellow on top). Unexpected cross-regulation of six MEK1 phospho-sites after EGF treatment was found by this investigation. It is yet unknown which specific kinases and phosphatases are engaged in this cross-regulation as well as the functions of the various MEK1 phosphorylation patterns .
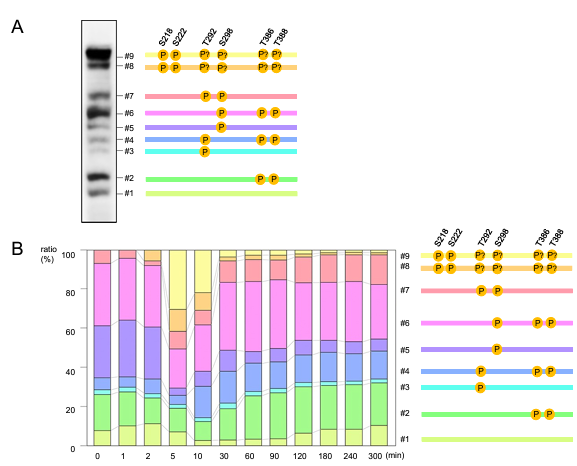
Figure 4: Example of multi-site analysis using Phos-tag gels. A. A431 cells were treated with EGF .B. Quantification of endogenous MEK1 phosphorylation.
One of the regulators of smooth muscle contraction, myosin-targeting subunit of myosin light chain phosphatase (MYPT1), has undergone a similar investigation of multi-site phosphorylations utilizing Phos-tag gels. It was possible to identify more than ten residues that were phosphorylated concurrently and to calculate the stoichiometry of phosphorylations. It's interesting to note that research on crosstalk between phospho-sites revealed that some phosphorylations prevented the phosphorylation of other sites.
The process of integrating complicated phosphorylation patterns caused by several kinases at the substrate level can be better understood with the use of phospho-tag gel analysis. A few recent instances are the kinases DYRK and GSK3 phosphorylating methylenetetrahydrofolate reductase on multiple sites, PKA and CaMKI phosphorylating and regulating the protein phosphatase PPM1H, CDK1 and CDK5 phosphorylating DDHD1, an intracellular phospholipase, on four phospho-sites, and cellular and viral kinases phosphorylating the cytomegaloviral mRNA Export Factor pUL69 .
In the realm of phosphoproteomics, phospho-tag acrylamide has become a potent instrument that provides a sensitive and selective method for the study of phosphorylated proteins. It is a priceless tool for researchers looking into kinase activity, post-translational changes, and cellular signaling networks because of its mass spectrometry compatibility and capacity to yield quantitative insights. Phos-tag acrylamide is anticipated to be essential in helping to decipher the phosphoproteome's intricacies and broaden our knowledge of molecular regulation of cells as proteomics develops.














Comments