Dienogest (Synonyms: STS 557) |
| Catalog No.GC15578 |
Dienogest(STS-557)는 COX-2, mPGES-1 및 아로마타제의 유전자 발현을 효과적으로 감소시키는 경구 활성 및 선택적 프로게스테론 수용체 작용제입니다.
Products are for research use only. Not for human use. We do not sell to patients.
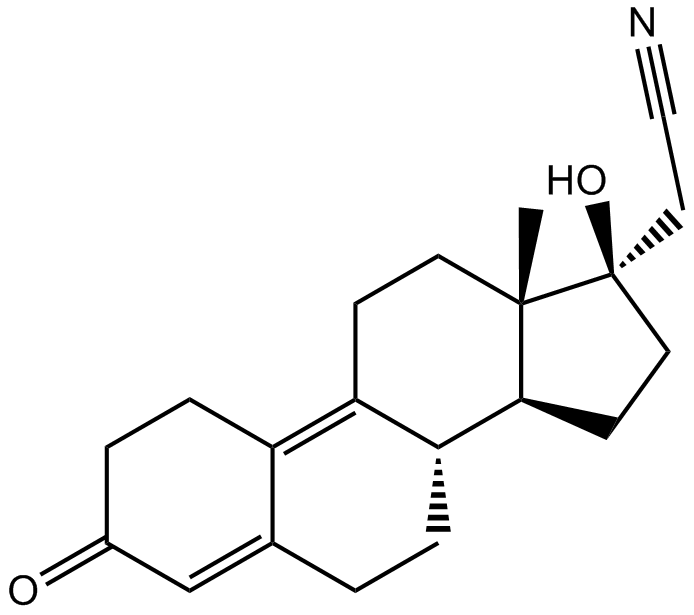
Cas No.: 65928-58-7
Sample solution is provided at 25 µL, 10mM.
Dienogest(STS-557) is a specific progesterone receptor agonist with potent oral endometrial activity and is used in the treatment of endometriosis. Target: progesterone receptor agonistDienogest is an orally active synthetic progesterone (or progestin). It is available for use as an oral contraceptive in combination with ethinylestradiol. It has antiandrogenic activity and as a result can improve androgenic symptoms. It is a non-ethinylated progestin which is structurally related to testosterone [1]. Complete sperm suppression was observed in rats sacrificed either 60 or 90 days after dienogest (DNG)+ testosterone undecanoate (TU) administration, for two injections at 45-day interval. The neutral α-glucosidase activity in these treated rats remained in the normal range. Germ cell loss due to apoptosis was frequently observed both after 60 or 90 days of combination treatment. Significant decline in serum gonadotropin and testosterone, both serum and intratesticular levels, were observed in the treated rats. Following stoppage of treatment (given at 45-day interval) after two (0 and 45 days) or three injections (0, 45 and 90 days), complete restoration of spermatogenesis was observed by 120 and 165 days, respectively [2].Clinical indications: Adenomyosis; EndometriosisFDA Approved Date: 1995 Toxicity: weight gain; increased blood pressure; breast tenderness and nausea
References:
[1]. Oettel M, et al. Effect of ethinyl estradiol-dienogest combination on serum androgen concentrations. Zentralbl Gynakol. 1997;119(12):597-606.
[2]. Meena R, et al. Extended intervention time and evaluation of sperm suppression by dienogest plus testosterone undecanoate in male rat. Contraception. 2012 Jan;85(1):113-21.
Average Rating: 5 (Based on Reviews and 2 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















