Iguratimod (Synonyms: IGU, T-614) |
| Catalog No.GC17419 |
A DMARD and COX-2 inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
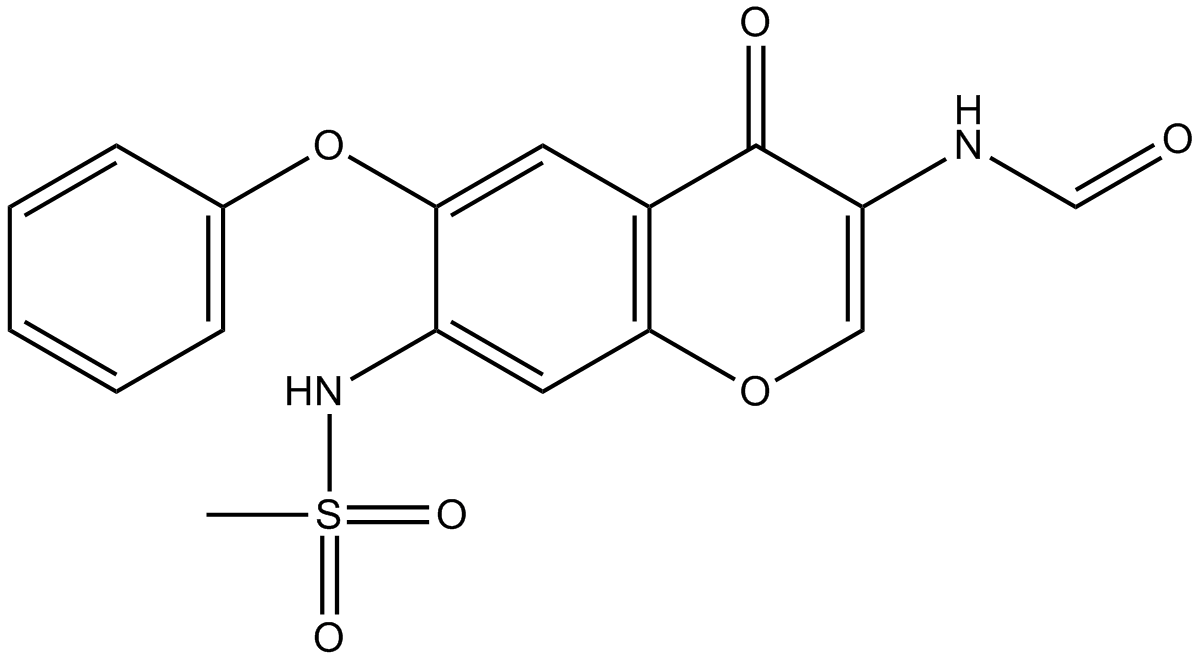
Cas No.: 123663-49-0
Sample solution is provided at 25 µL, 10mM.
IC50: 2.0 (hepatocyte-stimulating activities) and 6.6 μg/ml (immunoreactivities) for IL-6 release.
Iguratimod is one of a series of 4H-1-benzopyran-4-ones which has potent anti-inflammatory, antipyretic and analgesic activity. Iguratimod also inhibits the production of tumour necrosis factor and interleukin-1 (IL-1), IL-6, IL-8.
In vitro: Iguratimod inhibited the release of immunoreactive IL-1 beta from human monocytic cell line stimulated with lipopolysaccharides (LPS) in a dose-dependent manner (0.3-30 μg/ml). Northern blotting analysis using LPS-stimulated THP-1 cells indicated that the inhibitory effect of Iguratimod on IL-1 beta production is caused by the suppression of IL-1 beta mRNA expression [1].
In vivo: Administration of Iguratimod did not inhibit the tumor growth, but resulted in attenuation of cachexia symptoms. Furthermore, Iguratimod decreased the serum levels of IL-6, and also reduced its gene expression in the tumor tissues. In addition, exogenously administered IL-6 nullified the suppressive effect of Iguratimod [2].
Clinical trial: A 52-week clinical study of iguratimod in 394 Japanese patients with rheumatoid arthritis to evaluate the long-term safety of the drug was conducted. Iguratimod was administered orally at a daily dose of 25 mg for the first 4 weeks and 50 mg for the subsequent 48 weeks. The cumulative incidence of adverse events for 100 weeks was 97.6%. The cumulative incidence of adverse reactions was 65.3%; unfavorable symptoms and signs accounted for 33.2% of the reactions, and abnormal laboratory data changes accounted for 50.4% [3].
References:
[1] Tanaka K, Aikawa Y, Kawasaki H, Asaoka K, Inaba T, Yoshida C. Pharmacological studies on 3-formylamino-7-methylsulfonylamino-6-phenoxy-4H-1-benzopyran-4-one (T-614), a novel antiinflammatory agent. 4th communication: inhibitory effect on the production of interleukin-1 and interleukin-6. J Pharmacobiodyn. 1992;15(11):649-55.
[2] Tanaka K, Urata N, Mikami M, Ogasawara M, Matsunaga T, Terashima N, Suzuki H. Effect of iguratimod and other anti-rheumatic drugs on adenocarcinoma colon 26-induced cachexia in mice. Inflamm Res. 2007;56(1):17-23.
[3] Hara M, Abe T, Sugawara S, Mizushima Y, Hoshi K, Irimajiri S, Hashimoto H, Yoshino S, Matsui N, Nobunaga M. Long-term safety study of iguratimod in patients with rheumatoid arthritis. Mod Rheumatol. 2007;17(1):10-6.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















