Utibapril (FPL 63547) |
| Catalog No.GC32534 |
Utibapril(FPL 63547)은 항고혈압 활성을 갖는 안지오텐신 전환 효소(ACE) 억제제입니다.
Products are for research use only. Not for human use. We do not sell to patients.
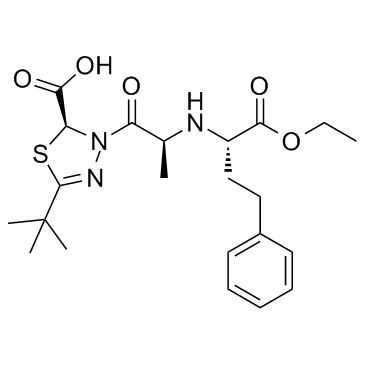
Cas No.: 109683-61-6
Sample solution is provided at 25 µL, 10mM.
Utibapril is an angiotensin-converting enzyme (ACE) inhibitor with antihypertensive activities.
Utibapril significantly inhibits plasma, renal, and vascular ACE but not ventricular ACE activity. Utibapril (2 μg/kg/day) significantly inhibits vascular ACE activity, and Utibapril (250 μg/kg/day) results in a significant inhibition of plasma ACE. Furthermore, angiotensin I-induced decreases in coronary flow in the isolated heart are significantly inhibited after treatment with the higher doses of utibapril[1]. FPL 63547 is rapidly and extensively excreted as the diacid in the bile but appeared in the urine in negligible amounts[2].
[1]. Buikema H, et al. Differential inhibition of plasma versus tissue ACE by utibapril: biochemical and functional evidence for inhibition of vascular ACE activity. J Cardiovasc Pharmacol. 1997 May;29(5):684-91. [2]. Carr RD, et al. Preferential biliary elimination of FPL 63547, a novel inhibitor of angiotensin-converting enzyme, in the rat. Br J Pharmacol. 1990 May;100(1):90-4.
Average Rating: 5 (Based on Reviews and 6 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















