Cefoperazone |
| Katalog-Nr.GC16501 |
Cefoperazon, ein halbsynthetisches Cephalosporin, hat ein breites Spektrum an antibakterieller AktivitÄt.
Products are for research use only. Not for human use. We do not sell to patients.
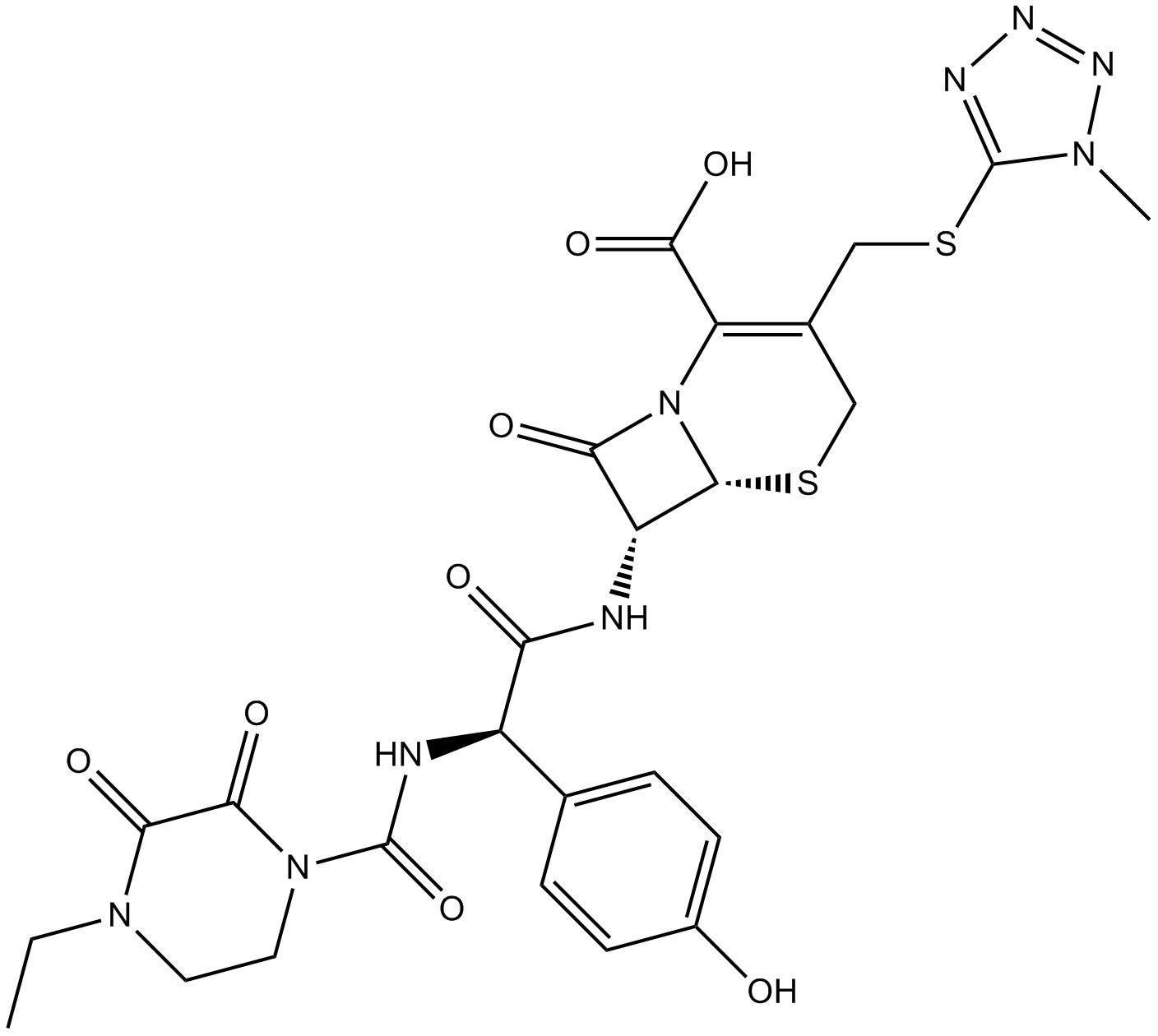
Cas No.: 62893-19-0
Sample solution is provided at 25 µL, 10mM.
Cefoperazone is a cephalosporin antibiotic which inhibits rMrp2-mediated [3H]E217βG uptake with an IC50 of 199 μM [1].
The inhibition profile for cefoperazone was biphasic with IC50, high and IC50, low values of 6.66 ± 3.23 μM and 3.88 ± 1.32 mM, respectively, indicating that cefoperazone may inhibit two binding sites that mediated [3H]E217βG transport [1]. Cefoperazone is a sterile, broad-spectrum, semisynthetic, parenteral cephalosporin antibiotic for intravenous or intramuscular administration. After intravenous administration of 2 g cefoperazone, levels in serum ranged from 202 μg/mL to 375 μg/mL depending on the period of drug administration. After intramuscular injection of 2 g of cefoperazone, the mean peak serum level was111 μg/mL at 1.5 hours. At 12 hours after dosing, mean serum levels were still 2 to 4 μg/mL.Cefoperazone is 90% bound to serum proteins.The apparent volume of distribution was 10 to 13L. The half-life of the drug varied from 1.6 to 2.4 hours, and the serum clearance was between 75 and 96 ml/min [2].
References:
[1]. Kato Y1,Takahara S,Kato S,Kubo Y,Sai Y,Tamai I,Yabuuchi H,Tsuji A. Involvement of multidrug resistance-associated protein 2 (Abcc2) in molecular weight-dependent biliary excretion of beta-lactam antibiotics.Drug Metab Dispos.2008 Jun;36(6):1088-96. doi: 10.1124/dmd.107.019125. Epub 2008 Mar 13.
[2]. Craig WA,Gerber AU. Pharmacokinetics of cefoperazone: a review. Drugs.1981;22Suppl 1:35-45.
Average Rating: 5 (Based on Reviews and 5 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















